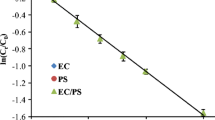
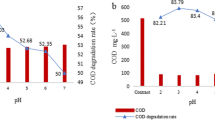
Abstract
Recently, persulfate (PS) has been applied to the oxidation of organic contaminants in wastewater, groundwater, and soil. However, PS requires activation by UV light, heat, transition metal, or pH control to be useful. In particular, transition metals are able to rapidly activate PS to sulfate radical. However, it is difficult to control the concentration of transition metal solution in an environmental setting. In this study, the potential of an electrochemical reaction using an iron anode to activate PS was investigated with phenol as a model contaminant. Based on Faraday’s law, Fe(II) generated by the electrochemical reaction was regularly supplied to the solution to activate PS to sulfate radical. The activation of PS was influenced by current intensity; however, excess Fe(II) decreased the oxidation rate of phenol because anodic oxidation-generated Fe(II) also scavenged sulfate radical. However, the electrochemical reaction using the iron anode could be readily controlled to supply an optimal amount of Fe(II) for activation of PS. Therefore, it is expected that this electrochemical process using an iron anode could be useful for the effective removal of phenol.





Similar content being viewed by others
References
Baek, K., Ciblak, A., Mao, X. H., Kim, E. J., & Alshawabkeh, A. (2013a). Iron anode mediated transformation of selenate in sand columns. Water Research, 47, 6538–6545.
Baek, K., Kasem, N., Ciblak, A., Vesper, D., Padilla, I., & Alshawabkeh, A. N. (2013b). Electrochemical removal of selenate from aqueous solutions. Chemical Engineering Journal, 215, 678–684.
Deng, D., Peng, L., Guan, M., & Kang, Y. (2014). Impact of activation methods on persulfate oxidation of methyl tert-butyl ether. Journal of Hazardous Materials, 264, 521–528.
Ehl, R. G., & Ihde, A. J. (1954). Faraday’s electrochemical laws and the determination of equivalent weights. Journal of Chemical Education, 31, 226.
Fan, G. P., Cang, L., Fang, G. D., & Zhou, D. M. (2014). Surfactant and oxidant enhanced electrokinetic remediation of a PCBs polluted soil. Separation and Purification Technology, 123, 106–113.
Ferrarese, E., Andreottola, G., & Oprea, I. A. (2008). Remediation of PAH-contaminated sediments by chemical oxidation. Journal of Hazardous Materials, 152, 128–139.
Huang, K. C., Couttenye, R. A., & Hoag, G. E. (2002). Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere, 49, 413–420.
Huling, S. G. & Pivetz, B. E. (2006). In situ chemical oxidation. EPA.
ITRC. (2005). Technical and regulatory guidance for in situ chemical oxidation of contaminated soil and groundwater. ITRC ISCO TEAM: Washington DC.
Kordkandi, S. A., & Forouzesh, M. (2014). Application of full factorial design for methylene blue dye removal using heat-activated persulfate oxidation. Journal of the Taiwan Institute of Chemical Engineers, 45, 2597–2604.
Liang, H. Y., Zhang, Y. Q., Huang, S. B., & Hussain, I. (2013). Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chemical Engineering Journal, 218, 384–391.
Liu, C. S., Shih, K., Sun, C. X., & Wang, F. (2012). Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Science of the Total Environment, 416, 507–512.
Marin, J. A., Hernandez, T., & Garcia, C. (2005). Bioremediation of oil refinery sludge by landfarming in semiarid conditions: influence on soil microbial activity. Environmental Research, 98, 185–195.
Namkoong, W., Hwang, E. Y., Park, J. S., & Choi, J. Y. (2002). Bioremediation of diesel-contaminated soil with composting. Environmental Pollution, 119, 23–31.
Son, H. S., Im, J. K., & Zoh, K. D. (2009). A Fenton-like degradation mechanism for 1,4-dioxane using zero-valent iron (Fe-0) and UV light. Water Research, 43, 1457–1463.
Stefan, M. I., & Bolton, J. R. (1998). Mechanism of the degradation of 1,4-dioxane in dilute aqueous solution using the UV hydrogen peroxide process. Environmental Science & Technology, 32, 1588–1595.
Strong, F. C. (1961). Faraday’s laws in one equation. Journal of Chemical Education, 38, 98.
Sutton, N. B., Grotenhuis, T., & Rijnaarts, H. H. M. (2014). Impact of organic carbon and nutrients mobilized during chemical oxidation on subsequent bioremediation of a diesel-contaminated soil. Chemosphere, 97, 64–70.
Tan, C. Q., Gao, N. Y., Chu, W. H., Li, C., & Templeton, M. R. (2012). Degradation of diuron by persulfate activated with ferrous ion. Separation and Purification Technology, 95, 44–48.
Tsai, T. T., Sah, J., & Kao, C. M. (2010). Application of iron electrode corrosion enhanced electrokinetic-Fenton oxidation to remediate diesel contaminated soils: a laboratory feasibility study. Journal of Hydrology, 380, 4–13.
Wang, C. W., & Liang, C. J. (2014). Oxidative degradation of TMAH solution with UV persulfate activation. Chemical Engineering Journal, 254, 472–478.
Watts, R. J., & Dilly, S. E. (1996). Evaluation of iron catalysts for the Fenton-like remediation of diesel-contaminated soils. Journal of Hazardous Materials, 51, 209–224.
Yuan, S. H., Liao, P., & Alshawabkeh, A. N. (2014). Electrolytic manipulation of persulfate reactivity by iron electrodes for trichloroethylene degradation in groundwater. Environmental Science & Technology, 48, 656–663.
Zazo, J. A., Casas, J. A., Mohedano, A. F., Gilarranz, M. A., & Rodriguez, J. J. (2005). Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environmental Science & Technology, 39, 9295–9302.
Acknowledgements
This research was supported by the R&D Center for Green Patrol Technologies through the R&D for Global Top Environmental Technologies funded by the Ministry of Environment, Republic of Korea (MOE), and partially supported by the National Research Foundation (Grant Number: NRF-2015R1D1A1A09060537).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, SM., Lee, SW., Jeon, PY. et al. Iron Anode-Mediated Activation of Persulfate. Water Air Soil Pollut 227, 462 (2016). https://doi.org/10.1007/s11270-016-3169-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3169-4