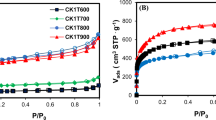
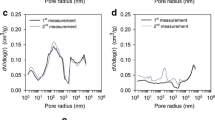
Abstract
The adsorption of ethyl acetate, a volatile organic compound, on activated carbons, synthesized from various precursors based on by-products and waste materials—polymer, biomass, coal tar pitch—was studied. The activated carbons were prepared by thermochemical treatment of the precursors, carbonization, and subsequent activation with water vapor. Surface and textural properties of obtained carbon adsorbents were characterized by low-temperature N2 adsorption, Boehm’s method, etc. The activated carbons are distinguished by relatively high surface area and developed pore structure. The adsorption investigations were performed with water solutions of ethyl acetate, and the obtained results fit well the Langmuir model, as well as the Freundlich model. All activated carbons demonstrated considerably high adsorption capacity in the range 160–450 mg/g. The obtained data indicate that the adsorption ability of activated carbon toward ethyl acetate depends on the surface area, and it increases with increasing the content of mesopores, where ethyl acetate molecules are preferably adsorbed.






Similar content being viewed by others
References
Ania, C. O., Parra, J.-B., Arenillas, A., Rubiera, F., Bandosz, T. J., & Pis, J. J. (2007). On the mechanism of reactive adsorption of dibenzothiophene on organic waste derived carbons. Applied Surface Science, 253, 5899–5903.
Asasian, N., Kaghazchi, T., & Soleimani, M. (2012). Elimination of mercury by adsorption onto activated carbon prepared from the biomass material. Journal of Industrial and Engineering Chemistry, 18, 283–289.
Bacaoui, A., Yaacoubi, A., Dahbi, A., Bennouna, C., Phan Tan Luu, R., Maldonado-Hodar, F. J., Rivera-Utrilla, J., & Moreno-Castilla, C. (2001). Optimization of conditions for the preparation of activate carbon from olive waste cakes. Carbon, 39, 425–432.
Bandosz, T. (2005). Activated carbon surfaces in environmental remediation. Interface Science and Technology. New York: Elsevier.
Bansal, R. C., & Goyal, M. (2005). Activated carbon adsorption. Boca Raton: Taylor & Francis.
Berenjian, A., Chan, N., & Malmiri, H. J. (2012). Volatile organic compounds removal methods: a review. American Journal of Biochemistry and Biotechnology, 8, 220–229.
Boehm, H. P. (2002). Surface oxides on carbon and their analysis: a critical assessment. Carbon, 40, 145–149.
Branton, P., Urita, K., & Kaneko, K. (2010). Ethyl acetate adsorption onto activated carbon. Adsorption Science and Technology, 28, 895–902.
Budinova, T., Krzesinska, M., Tsyntsarski, B., Zachariasz, J., & Petrova, B. (2008). Activated carbon produced from bamboo pellets for removal of arsenic(III) ions from water. Bulgarian Chemical Communications, 40, 166–172.
Budinova, T., Savova, D., Tsyntsarski, B., Ania, C. O., Cabal, B., Parra, J.-B., & Petrov, N. (2009). Biomass waste-derived activated carbon for the removal of arsenic and manganese ions from aqueous solution. Applied Surface Science, 255, 4650–4657.
Dolidovich, A. F., Akhremkova, G. S., & Efremtsev, V. S. (1999). Novel technologies of VOC decontamination in fixed, moving and fluidized catalyst-adsorbent beds. The Canadian Journal of Chemical Engineering, 77, 342–355.
Ekinci, E., Ferhat Yardim, M., Razvigorova, M., Minkova, V., Goranova, M., Petrov, N., & Budinova, T. (2002). Characterization of liquid products from pyrolysis of subbituminous coals. Fuel Processing Technology, 77–78, 309–315.
Gales, L., Mendes, A., & Costa, C. (2000). Hysteresis in the cyclic adsorption of acetone, ethanol and ethyl acetate on activated carbon. Carbon, 38, 1083–1088.
Gergova, K., Petrov, P., & Eser, S. (1994). Adsorption properties and microstructure of activated carbons produced from agricultural by-products by steam pyrolysis. Carbon, 32, 693–702.
Husson, J., Dehaudt, J., & Guyard, L. (2015). Furfuraldehyde: from plant harvest to light harvest? Journal of Environmental Chemical Engineering, 3, 2292–2300.
Hwang, K. S., Choi, D. K., Gong, S. Y., & Cho, S. Y. (1997). Adsorption and thermal regeneration of methylene chloride vapor on an activated carbon bed. Chemical Engineering Science, 52, 1111–1123.
Khan, F. I., & Ghoshal, A. K. (2000). Removal of volatile organic compounds from polluted air. Journal of Loss Prevention in the Process Industries, 13, 527–545.
Khezami, L., Chetouani, A., Taouk, B., & Capart, B. (2005). Production and characterization of activated carbon from wood components in powdered: cellulose, lignin, xylan. Powder Technology, 157, 48–56.
Laszlo, K., Bota, A., Nagy, V., & Cabasso, I. (1999). Porous carbon from polymer waste materials. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 151, 311–320.
Manjare, S. D., & Ghoshal, A. K. (2005). Studies on dynamic adsorption behavior of ethyl acetate on molecular sieves. The Canadian Journal of Chemical Engineering, 83, 232–241.
Manjare, S. D., & Ghoshal, A. K. (2006a). Adsorption equilibrium studies for ethyl acetate vapour and E-Merck 13X molecular sieve system. Separation and Purification Technology, 51, 118–125.
Manjare, S. D., & Ghoshal, A. (2006b). Comparison of adsorption of ethyl acetate on activated carbon and molecular sieves 5A and 13X. Journal of Chemical & Engineering Data, 51, 1185–1189.
Minkova, V., Razvigorova, M., Bjornbom, E., Zanzi, R., Budinova, T., & Petrov, N. (2001). Effect of water vapour and biomass nature on the yield and quality of the pyrolysis products from biomass. Fuel Processing Technology, 70, 53–61.
Ohaa, N. V., Igbokwe, P. K., Obiora-Okafo, I. A., Ejikeme, E. M., & Government, R.M. (2013a). Adsorption of ethyl acetate from ethyl acetate-water mixture using activated clay. International Journal of Multidisciplinary Science and Engineering, 4, 10–15.
Ohaa, N. V., Igbokwe, P. K., Obiora-Okafo, L. A., & Government, R.M. (2013b). Uptake of ethyl acetate from ethyl acetate-water mixture using activated carbon. International Journal of Multidisciplinary Science and Engineering, 4, 35–40.
Papirer, E., Li, S., & Donnet, J.-B. (1987). Contribution to the study of basic surface groups on carbons. Carbon, 25, 243–247.
Park, S.-J., & Jung, W.-Y. (2002). Preparation and structural characterization of activated carbons based on polymer resin. Journal of Colloid and Interface Science, 250, 196–200.
Petrov, N., Budinova, T., Razvigorova, M., Parra, J.-B., & Galiatsatou, P. (2008). Conversion of olive wastes to volatiles. Biomass and Bioenergy, 32, 1303–1310.
Petrov, N., Budinova, T., Tsyntsarski, B., Petrova, B., Teodosiev, D., & Boncheva, N. (2010). Synthesis of nanoporous carbon from plant wastes and coal treatment pyrolysis. Bulgarian Chemical Communications, 42, 16–19.
Petrova, B., Budinova, T., Tsyntsarski, B., Petrov, N., Ania, C. O., & Parra, J.-B. (2010). Phenol adsorption on activated carbons with different structure and properties. Bulgarian Chemical Communications, 42, 141–146.
Petrova, B., Tsyntsarski, B., Budinova, T., Petrov, N., Ania, C. O., Parra, J. B., Mladenov, M., & Tzvetkov, P. (2011). Synthesis of nanoporous carbons from mixtures of coal tar pitch and furfural and their application as electrode materials. Fuel Processing Technology, 91, 1710–1716.
Ryu, Z., Rong, H., Zheng, J., Wang, M., & Zhang, B. (2002). Microstructure and chemical analysis of PAN-based activated carbon fibers prepared by different activation methods. Carbon, 40, 1144–1147.
Saka, C. (2012). BET, TG–DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. Journal of Analytical and Applied Pyrolysis, 95, 21–24.
San Miguel, G., Fowler, G. D., & Sollars, C. J. (2003). A study of the characteristics of activated carbons produced by stream and carbon dioxide activation of waste tyre rubber. Carbon, 41, 1009–1016.
Savova, D., Apak, E., Ekinci, E., Ferhat Yardim, M., Petrov, N., Budinova, T., Razvigorova, M., & Minkova, V. (2001). Biomass conversion to carbon adsorbents and gas. Biomass and Bioenergy, 21, 133–142.
Shalaby, C. S., Ucak-Astarliog Iu, M. G., Artok, L., & Sarici, C. (2006). Preparation and characterization of activated carbons by one-step pyrolysis/activation from apricot stones. Microporous and Mesoporous Materials, 88, 126–134.
Tamai, H., Kouzu, M., & Yasuda, H. (2003). Preparation of highly mesoporous and high surface area activated carbons from vinylidene chloride copolymer containing yttrium acetylacetonate. Carbon, 41, 1678–1681.
Tan, C. S., & Liou, D. C. (1988). Desorption of ethyl acetate from activated carbon by supercritical carbon dioxide. Industrial & Engineering Chemistry Research, 27, 988–991.
Tsyntsarski, B., Petrova, B., Budinova, T., Petrov, N., Teodosiev, D., Sarbu, A., Sandu, T., Ferhat Yardim, M., & Sirkecioglu, A. (2014). Removal of detergents from water by adsorption on activated carbons obtained from various precursors. Desalination and Water Treatment, 52, 3445–3452.
Acknowledgments
The authors gratefully appreciate the funding by the Bulgarian Ministry of Education and Science under Project DFNI E 02-2 (12.12.2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoycheva, I.G., Tsyntsarski, B.G., Petrova, B.N. et al. Adsorption of Ethyl Acetate from Water by Nanoporous Carbon Prepared from Waste Materials. Water Air Soil Pollut 227, 452 (2016). https://doi.org/10.1007/s11270-016-3099-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3099-1