Abstract
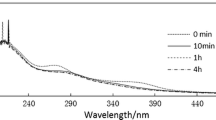
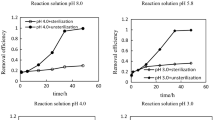
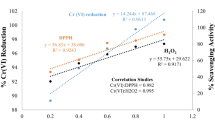
The effect of Fe(III) on Cr(VI) reduction by organic reducing substances in sugarcane molasses was investigated under different conditions [i.e., Fe(III) concentration, pH, and temperature] using batch experiments. Results indicated that Fe(III) can accelerate Cr(VI) reduction by sugarcane molasses over a wide pH range. The catalytic mechanism of the reaction involved the formation of organic reducing substance complexes with both Fe(III) and Cr(VI) that decrease the reaction activation energy of Cr(VI) reduction and accelerate electron transfer between Cr(VI) and organic reducing substances. The reaction could be described by a pseudo-first-order kinetic model with respect to Cr(VI) concentration. Increasing the Fe(III) concentration could promote Cr(VI) reduction. At pH 2.5, 3.0, 3.5, 4.0, 5.6, and 8.0, the initial reaction rates (vinitial) increased by 0.68, 0.84, 1.38, 1.39, 0.89, and 0.29 times, respectively, in the presence of Fe(III) compared with those obtained without Fe(III). The vinitial increased by 0.87 times in the presence of Fe(III) compared with that without Fe(III) at 10 °C (pH 2.5).






Similar content being viewed by others
References
Babu, P. S. S., Khan, Z., & Kabir-ud-Din. (2004). Electron transfer reaction in the chromium(VI)-manganese(II) system in the presence of ethylenediaminetetraacetic acid (EDTA). Transition Metal Chemistry, 29(8), 885–892.
Bond, D. L., & Fendorf, S. (2003). Kinetics and structural constraints of chromate reduction by green rusts. Environmental Science & Technology, 37(12), 2750–2757.
Brodie, E. L., Joyner, D. C., Faybishenko, B., Conrad, M. E., Rios-Velazquez, C., Malave, J., Martinez, R., Mork, B., Willett, A., Koenigsberg, S., Herman, D. J., Firestone, M. K., & Hazen, T. C. (2011). Microbial community response to addition of polylactate compounds to stimulate hexavalent chromium reduction in groundwater. Chemosphere, 85(4), 660–665.
Chauhan, D., Dwivedi, J., & Sankararamakrishnan, N. (2014). Facile synthesis of smart biopolymeric nanofibers towards toxic ion removal and disinfection control. RSC Advances, 4(97), 54694–54702.
Chen, N., Lan, Y., Wang, B., & Mao, J. (2013). Reduction of Cr (VI) by organic acids in the presence of Al (III). Journal of Hazardous Materials, 260, 150–156.
Chen, J., Chen, R., & Hong, M. (2015a). Influence of pH on hexavalent chromium reduction by Fe(II) and sulfide compounds. Water Science and Technology, 72, 22–28.
Chen, Z. F., Zhao, Y. S., Zhang, J. W., & Bai, J. (2015b). Mechanism and kinetics of hexavalent chromium chemical reduction with sugarcane molasses. Water, Air, and Soil Pollution, 226, 363.
Chrysochoou, M., & Ting, A. (2011). A kinetic study of Cr(VI) reduction by calcium polysulfide. Science of the Total Environment, 409(19), 4072–4077.
Chrysochoou, M., Johnston, C. P., & Dahal, G. (2012). A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. Journal of Hazardous Materials, 201–202, 33–42.
Field, E. K., Gerlach, R., Viamajala, S., Jennings, L. K., Peyton, B. M., & Apel, W. A. (2013). Hexavalent chromium reduction by Cellulomonas sp strain ES6: the influence of carbon source, iron minerals, and electron shuttling compounds. Biodegradation, 24(3), 437–450.
Fu, F., Dionysiou, D. D., & Liu, H. (2014). The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. Journal of Hazardous Materials, 267C, 194–205.
Guan, Y., Tang, Q., Fu, X., Yu, S., Wu, S., & Chen, M. (2014). Preparation of antioxidants from sugarcane molasses. Food Chemistry, 152, 552–557.
Han, J., Bu, X., Zhou, D., Zhang, H., & Yang, B. (2014). Discriminating Cr(III) and Cr(VI) using aqueous CdTe quantum dots with various surface ligands. RSC Advances, 4(62), 32946–32952.
Hashim, M. A., Mukhopadhyay, S., Sahu, J. N., & Sengupta, B. (2011). Remediation technologies for heavy metal contaminated groundwater. Journal of Environmental Management, 92(10), 2355–2388.
Henderson, A. D., & Demond, A. H. (2013). Permeability of iron sulfide (FeS)-based materials for groundwater remediation. Water Research, 47(3), 1267–1276.
Hosseini, M. S., & Belador, F. (2009). Cr(III)/Cr(VI) speciation determination of chromium in water samples by luminescence quenching of quercetin. Journal of Hazardous Materials, 165(1–3), 1062–1067.
Hug, S. J., Laubscher, H. U., & James, B. R. (1997). Iron(III) catalyzed photochemical reduction of chromium(VI) by oxalate and citrate in aqueous solutions. Environmental Science and Technology, 37, 160–170.
Hynes, M. J., & Coinceanainn, M. O. (2001). The kinetics and mechanisms of the reaction of iron(III) with gallic acid, gallic acid methyl ester and catechin. Journal of Inorganic Biochemistry, 85, 131–142.
Khan, Z., Hashmi, A. A., Ahmed, L., & Hao, M. M. (1998). Kinetics and mechanism of chromic acid oxidation of oxalic acid in absence and presence of different acid media. A kinetic study. International Journal of Chemical Kinetics, 30, 335–340.
Krishna, K. R., & Philip, L. (2005). Bioremediation of Cr(VI) in contaminated soils. Journal of Hazardous Materials, 121(1–3), 109–117.
Lee, S. E., Lee, J. U., Chon, H. T., & Lee, J. S. (2008). Reduction of Cr(VI) by indigenous bacteria in Cr-contaminated sediment under aerobic condition. Journal of Geochemical Exploration, 96, 144–147.
Li, C., Lan, Y. Q., & Deng, B. L. (2007). Catalysis of manganese(II) on chromium(VI) reduction by citrate. Pedosphere, 17(3), 318–323.
Lin, Y. T., & Huang, C. P. (2008). Reduction of chromium(VI) by pyrite in dilute aqueous solutions. Separation and Purification Technology, 63, 191–199.
Loyaux-Lawniczak, S., Refait, P., Ehrhardt, J. J., Lecomte, P., & Genin, J. M. R. (2000). Trapping of Cr by formation of ferrihydrite during the reduction of chromate ions by Fe(II)-Fe(III) hydroxysalt green rusts. Environmental Science & Technology, 34(3), 438–443.
Ludwig, R. D., Su, C. M., Lee, T. R., Wilkin, R. T., Acree, S. D., Ross, R. R., & Keeley, A. (2007). In situ chemical reduction of Cr(VI) in groundwater using a combination of ferrous sulfate and sodium dithionite: a field investigation. Environmental Science & Technology, 41(15), 5299–5305.
Michael, J., & Hynes, M. O. C. (2001). The kinetics and mechanisms of the reaction of iron(III) with gallic acid, gallic acid methyl ester and catechin. Journal of Inorganic Biochemistry, 85, 131–142.
Michailides, M. K., Tekerlekopoulou, A. G., Akratos, C. S., Coles, S., Pavlou, S., & Vayenas, D. V. (2014). Molasses as an efficient low-cost carbon source for biological Cr(VI) removal. Journal of Hazardous Materials, 281, 95–105.
Okello, V. A., Mwilu, S., Noah, N., Zhou, A., Chong, J., Knipfing, M. T., Doetschman, D., & Sadik, O. A. (2012). Reduction of hexavalent chromium using naturally-derived flavonoids. Environmental Science & Technology, 46(19), 10743–10751.
Pagnanelli, F., Viggi, C. C., Cibati, A., Uccelletti, D., Toro, L., & Palleschi, C. (2012). Biotreatment of Cr(VI) contaminated waters by sulphate reducing bacteria fed with ethanol. Journal of Hazardous Materials, 199–200, 186–192.
Patterson, R. R., Fendorf, S., & Fendorf, M. (1997). Reduction of hexavalent chromium by amorphous iron sulfide. Environmental Science & Technology, 31(7), 2039–2044.
Qin, G., Mcguire, M. J., Blute, N. K., Seidel, C., & Fong, L. (2005). Hexavalent chromium removal by reduction with ferrous sulfate coagulation, and filtration: a pilot-scale study. Environmental Science and Technology, 39, 6321–6327.
Qiu, X., Fang, Z., Yan, X., Cheng, W., & Lin, K. (2013). Chemical stability and toxicity of nanoscale zero-valent iron in the remediation of chromium-contaminated watershed. Chemical Engineering Journal, 220, 61–66.
Sarkar, B., Naidu, R., Krishnamurti, G. S. R., & Megharaj, M. (2013). Manganese(II)-catalyzed and clay-minerals-mediated reduction of chromium(VI) by citrate. Environmental Science & Technology, 47(23), 13629–13636.
Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxidants and Antioxidants, Part A, 299, 152–178.
Somasundaram, V., Philip, L., & Bhallamudi, S. M. (2009). Experimental and mathematical modeling studies on Cr(VI) reduction by CRB, SRB and IRB, individually and in combination. Journal of Hazardous Materials, 172(2–3), 606–617.
Somasundaram, V., Philip, L., & Bhallamudi, S. M. (2011). Laboratory scale column studies on transport and biotransformation of Cr(VI) through porous media in presence of CRB, SRB and IRB. Chemical Engineering Journal, 171(2), 572–581.
Standard Methods for the Examination of Water and Wastewater, 20th ed., (1998). American Water Works Association and Water Pollution Control Federation, Washington D.C.
Su, C. M., & Ludwig, R. D. (2005). Treatment of hexavalent chromium in chromite ore processing solid waste using a mixed reductant solution of ferrous sulfate and sodium dithionite. Environmental Science & Technology, 39(16), 6208–6216.
Sugiyama, T., Sugito, H., Mamiya, K., Suzuki, Y., Ando, K., & Ohnuki, T. (2012). Hexavalent chromium reduction by an actinobacterium Flexivirga alba ST13(T) in the family Dermacoccaceae. Journal of Bioscience and Bioengineering, 113(3), 367–371.
Sun, J., Mao, J. D., Gong, H., & Lan, Y. (2009). Fe(III) photocatalytic reduction of Cr(VI) by low-molecular-weight organic acids with α-OH. Journal of Hazardous Materials, 168(2–3), 1569–1574.
US EPA (2011). Molasses Injections at Selma Superfund Site Result in Multiple Environmental and Economic Benefits. http://www.epa.gov/region9/cleanup-clean-air/selma.html.
US EPA (2003). Molasses-Based Microbial Precipitation Used Successfully for Chromium Reduction. http://www.epa.gov/tio/download/newsltrs/tnandt0703.pdf.
Yalçın Tepe, A. (2014). Toxic Metals: Trace Metals – Chromium, Nickel, Copper, and Aluminum. Encyclopedia of Food Safety. Y. Motarjemi (pp. 356–362). Waltham: Academic.
Acknowledgments
This research project was supported by College Innovation Ability Promotion Projects of Beijing Municipal Education Commission (Grant No. TJSHG201310772028). The authors are grateful to the Analysis and Testing Foundation of the Key Laboratory of Groundwater Resources and Environment, Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, ZF., Zhao, YS. & Li, Q. Influence of Fe(III) on Cr(VI) Reduction by Organic Reducing Substances from Sugarcane Molasses. Water Air Soil Pollut 227, 19 (2016). https://doi.org/10.1007/s11270-015-2678-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2678-x