Abstract
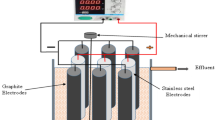
The aim of this study is to develop a process that combines electrocoagulation and electroperoxidation (EC-EP) and to evaluate its performance in treating domestic wastewaters (DWW). Electrolysis was performed using a parallelepipedic electrolytic cell (0.5 L) containing one sacrificial anode (mild steel or aluminum) and one cathode (vitreous carbon). The effects of the treatment time, current density, and type of anode electrode on the process performance were examined. The experimental results revealed that a current density of 4.0 mA cm−2 was beneficial for DWW treatment. There was a decrease in the chemical oxygen demand (COD), suspended solid (SS), turbidity, color, and total phosphorus (Ptot) by 67 ± 9, 98 ± 2, 55 ± 10, 61 ± 9, and 97 ± 0 %, respectively, for a treatment time of 60 min in the electrolysis cell in the presence of mild steel (anode) and vitreous carbon (cathode) electrodes. The process was also determined to be effective for removing pathogens (99 ± 1 % removal), such as fecal coliform (the log-inactivation was higher than 2 units).


Similar content being viewed by others
References
Abegglen, C., Ospelt, M., & Siegrist, H. (2008). Biological nutrient removal in a small-scale MBR treating household wastewater. Water Research, 42, 338–346.
Asselin, M., Drogui, P., Benmoussa, H., & Blais, J.-F. (2008). Effectiveness of electrocoagulation process in removing organic compounds from slaughterhouse wastewater using monopolar and bipolar electrolytic cells. Chemosphere, 72, 1727–1733.
Brillas, E., & Casado, J. (2002). Aniline degradation by Electro-Fenton® and peroxi-coagulation processes using a flow reactor for wastewater treatment. Chemosphere, 47, 241–248.
Brillas, E., Arias, C., Cabot, P., Centellas, F., Garrido, J., & Rodríguez, R. (2006). Degradation of organic contaminants by advanced electrochemical oxidation methods. Portugaliae Electrochimica Acta, 24, 159.
Brillas, E., Sirés, I., & Oturan, M. A. (2009). Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chemical Reviews, 109, 6570–6631.
CEAEQ. (2003). Détermination de la demande chimique en oxygène dans les effluents: méthode de reflux en système fermé suivi d’un dosage par colorimétrie avec le bichromate de potassium, MA. 315-DCO 1.0 (p. 14). Québec: Centre d’Expertise en Analyse Environnementale du Québec, Ministère de l’Environnement du Québec.
CEAEQ (2012a) Détermination de la couleur vraie dans l’eau : méthode par spectrophotométrie UV-visible avec le platino-cobalt, MA. 103-Col. 2.0, Rév. 2. Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs du Québec, 2012a, 8 p
CEAEQ: 2012b, Détermination des solides en suspension totaux et volatils : méthode gravimétrique, MA. 115 -S.S. 1.2, Rév. 1. Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs du Québec, Qc, 2012b, Canada, p. 13.
Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38, 11–41.
Daghrir, R., Drogui, P., François Blais, J., & Mercier, G. (2012). Hybrid process combining electrocoagulation and electro-oxidation processes for the treatment of restaurant wastewaters. Journal of Environmental Engineering, 138, 1146–1156.
Daghrir, R., Drogui, P., & El Khakani, M. (2013). Photoelectrocatalytic oxidation of chlortetracycline using Ti/TiO 2 photo-anode with simultaneous H2O2 production. Electrochimica Acta, 87, 18–31.
Drogui, P., Elmaleh, S., Rumeau, M., Bernard, C., & Rambaud, A. (2001). Hydrogen peroxide production by water electrolysis: application to disinfection. Journal of Applied Electrochemistry, 31, 877–882.
Flasche, K (2002) Possible applications and performance of small scale wastewater treatment plants, Dissertation of the Institute for Urban Water Management and Waste Technology, University of Hanover, 2002.
Flox, C., Cabot, P.-L., Centellas, F., Garrido, J. A., Rodríguez, R. M., Arias, C., & Brillas, E. (2007). Solar photoelectro-Fenton degradation of cresols using a flow reactor with a boron-doped diamond anode. Applied Catalysis B: Environmental, 75, 17–28.
Garrido, J. A., Brillas, E., Cabot, P. L., Centellas, F., Arias, C., & Rodríguez, R. M. (2007). Mineralization of drugs in aqueous medium by advanced oxidation processes. Portugaliae Electrochimica Acta, 25, 19.
Golder, A., Samanta, A., & Ray, S. (2007). Removal of Cr 3+ by electrocoagulation with multiple electrodes: bipolar and monopolar configurations. Journal of Hazardous Materials, 141, 653–661.
Guinea, E., Garrido, J. A., Rodríguez, R. M., Cabot, P.-L., Arias, C., Centellas, F., & Brillas, E. (2010). Degradation of the fluoroquinolone enrofloxacin by electrochemical advanced oxidation processes based on hydrogen peroxide electrogeneration. Electrochimica Acta, 55, 2101–2115.
Guivarch, E., Oturan, N., & Oturan, M. A. (2003). Removal of organophosphorus pesticides from water by electrogenerated Fenton’s reagent. Environmental Chemistry Letters, 1, 165–168.
Isarain-Chávez, E., Garrido, J. A., Rodríguez, R. M., Centellas, F., Arias, C., Cabot, P. L., & Brillas, E. (2011). Mineralization of metoprolol by electro-Fenton and photoelectro-Fenton processes. The Journal of Physical Chemistry. A, 115, 1234–1242.
Lahkimi, A., Oturan, M. A., Oturan, N., & Chaouch, M. (2007). Removal of textile dyes from water by the electro-Fenton process. Environmental Chemistry Letters, 5, 35–39.
Mameri, N., Yeddou, A., Lounici, H., Belhocine, D., Grib, H., & Bariou, B. (1998). Defluoridation of septentrional Sahara water of North Africa by electrocoagulation process using bipolar aluminium electrodes. Water Research, 32, 1604–1612.
MDDEP. (2008). Guide technique sur le traitement des eaux usées des résidences isolées. Québec: Ministère du Développement Durable, de l’Environnement et des Parcs du Québec, 2008. 23 p.
Meuler, S., Paris, S., & Hackner, T. (2008). Membrane bio-reactors for decentralized wastewater treatment and reuse. Water Science and Technology, 58, 285–294.
Meunier, N., Drogui, P., Montané, C., Hausler, R., Blais, J.-F., & Mercier, G. (2006). Heavy metals removal from acidic and saline soil leachate using either electrochemical coagulation or chemical precipitation. Journal of Environmental Engineering, 132, 545–554.
Millet, M. (1992). L’oxygene et les radicaux libres. Bios, 23(3), 45–50.
Özcan, A., Şahin, Y., & Oturan, M. A. (2008). Removal of propham from water by using electro-Fenton technology: kinetics and mechanism. Chemosphere, 73, 737–744.
Panizza, M., & Cerisola, G. (2009). Electro-Fenton degradation of synthetic dyes. Water Research, 43, 339–344.
Sigler, P. B., & Masters, B. (1957). The hydrogen peroxide-induced Ce*(III)-Ce (IV) exchange System1. Journal of the American Chemical Society, 79, 6353–6357.
Wang, C.-T., Hu, J.-L., Chou, W.-L., & Kuo, Y.-M. (2008). Removal of color from real dyeing wastewater by electro-Fenton technology using a three-dimensional graphite cathode. Journal of Hazardous Materials, 152, 601–606.
Wang, C.-T., Chou, W.-L., Chen, L.-S., & Chang, S.-Y. (2009). Silica particles settling characteristics and removal performances of oxide chemical mechanical polishing wastewater treated by electrocoagulation technology. Journal of Hazardous Materials, 161, 344–350.
WERF (2007) Influent constituent characteristics of the modern waste stream from single sources: literature review. Water Environment Research Foundation, ISBN 1-84339-773-0. Report 04-Dec-1a., 2007.
Zarei, M., Niaei, A., Salari, D., & Khataee, A. (2010). Application of response surface methodology for optimization of peroxi-coagulation of textile dye solution using carbon nanotube–PTFE cathode. Journal of Hazardous Materials, 173, 544–551.
Zhou, M., Yu, Q., & Lei, L. (2008). The preparation and characterization of a graphite–PTFE cathode system for the decolorization of CI acid Red 2. Dyes and Pigments, 77, 129–136.
Zhu, B., Clifford, D. A., & Chellam, S. (2005). Comparison of electrocoagulation and chemical coagulation pretreatment for enhanced virus removal using microfiltration membranes. Water Research, 39, 3098–3108.
Acknowledgments
Sincere thanks are extended to the National Sciences and Engineering Research Council of Canada and Premier Tech for their financial contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
SM 1
The evolution of H2O2 concentrations within the electrolytic cell when using an Fe, Al, or Ti/IrO2 electrode at the anode and a current density of 4 mA cm-2. (DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Senghor, F., Drogui, P. & Seyhi, B. A Combined Electrocoagulation-Electroperoxidation Process for the Tertiary Treatment of Domestic Wastewaters. Water Air Soil Pollut 226, 373 (2015). https://doi.org/10.1007/s11270-015-2637-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2637-6