Abstract
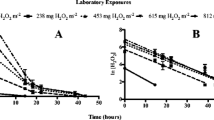
Algaecides are commonly used to control noxious algal growths in water resources. In order to make accurate predictions about responses of target and non-target species to algaecide exposures, reliable methods are needed to confirm exposures in the laboratory and the field. The focus of this research was to evaluate the I3 − method for measuring hydrogen peroxide (H2O2) exposures associated with applications of a sodium carbonate peroxyhydrate (SCP)-based algaecide. To meet this overall objective, method detection limits, interferences from field waters (turbidity, color, and algal cell density), and storage stability of samples were measured. The method detection limits were 0.2 (p < 0.0001) and 0.25 mg H2O2/L (p = 0.0002) for laboratory and field waters, respectively. The upper method detection limit was 7.5 mg H2O2/L for both waters. Turbidity (25.3 NTU) and color of field-collected water did not interfere with measurements. Algal cell densities of 104, 105, and 106 cells/mL of two planktonic algal species in laboratory water did not interfere with measurements. Measurements of samples stored in dark refrigeration and on ice remained stable over 4 days, while measurements of samples stored in direct sunlight in ambient temperatures were altered after 1 day. The I3 − method accurately measured H2O2 exposures in the laboratory water, field water, and laboratory water containing planktonic algae used in this experiment within the range of concentrations that would be applied in a field setting. These data demonstrated that this method has utility for confirming exposures after laboratory and field treatments of SCP-based algaecides.





Similar content being viewed by others
References
Applied Biochemists (AB). (2007). Material safety data sheets. Milwaukee: Laporte Water Technologies and Biochem.
Bishop, W. M., & Rodgers, J. H., Jr. (2011). Responses of Lyngbya magnifica Gardner to an algaecide exposure in the laboratory and field. Ecotoxicol Environ Saf, 74, 1832–1838.
Brezonik, P. L., & Arnold, W. A. (2011). Water chemistry: An introduction to the chemistry of natural and engineered aquatic system. New York: Oxford University Press.
Briand, J. F., Jacquet, S., Bernard, C., & Humbert, J. F. (2003). Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems. Vet Res, 34, 361–377.
Budavari, S., O’neil, M. J., Smith, A., & Heckelman, P. E. (Eds.). (1989). The merck index (11th ed.). New York: Chapman & Hall.
Christman, R. F., & Ghassemi, M. (1966). Chemical nature of organic color in water. American Water Works Association, 58, 723–741.
Drabkova, M., Admiraal, W., & Marsalek, B. (2007). Combined exposure to hydrogen peroxide and light: selective effects on cyanobacteria, green algae, and diatoms. Environ Sci Technol, 41, 309–314.
Fisher Scientific. (2009). MSDS, hydrogen peroxide (20–40%). Fair Lawn, NJ.
Getsinger, K., Dibble, E., Rodgers, J., Spencer, D., & Madsen, J. D. (2014). Benefits of controlling nuisance aquatic plants and algae in the United States. Council for Agricultural Science and Technology Issue Paper.
Human & Environmental Risk Assessment (HERA). (2002). Sodium percarbonate. <http://www.heraproject.com/files/6-F-04-HERA%20percarbonate%20full%20web%20wd.pdf> Accessed 28 May 2012.
Jain, V. K., & Bajaj, A. (2004). A textbook of design of electrical installations. Daryaganj: Laxmi Publications.
Jakob, H., Leininger, S., Lehmann, T., Jacobi, S., & Gutewort, S. (2012). Peroxo compounds, inorganic. Ullmann’s Encyclopedia of Industrial Chemistry, 26, 293–324.
Klassen, N. V., Marchington, D., & McGowan, H. C. E. (1994). H2O2 determination by the I3 − method and by KMnO4 titration. Anal Chem, 66, 2921–2925.
Martin, J. F. (1992). The use of sodium carbonate peroxyhydrate to treat off-flavor in commercial catfish ponds. Water Sci Technol, 25, 315–321.
Newman, L. (Ed.). (1993). Measurement challenges in atmospheric chemistry. Washington: American Chemical Society.
Nogueria, R. F. P., Oliveira, M. C., & Paterlini, W. C. (2005). Simple and fast spectrophotometric determination of H2O2 in photo-fenton reactions using metavanadate. Talanta, 66, 86–91.
Organization for Economic Cooperation and Development (OECD) (2006). Screening Information Data Set (SIDS): Sodium percarbonate. Paris, France.
Paerl, H. W. (1988). Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol Oceanogr, 33, 823–847.
Quimby, P. C., Jr., Kay, S. H., & Ouzts, J. D. (1988). Sodium carbonate peroxyhydrate as a potential algaecide. Journal of AquaticPlant Management, 26, 67–68.
Rodgers, J. H., Jr., Johnson, B. M., & Bishop, W. M. (2010). Comparison of three algaecides for controlling the density of Prymnesium parvum. J Am Water Works Assoc, 46, 153–160.
Tanner, P. A., & Wong, A. Y. S. (1998). Spectrophotometric determination of hydrogen peroxide in rainwater. Anal Chim Acta, 370, 279–287.
United States Environmental Protection Agency (USEPA) (2002). Biopesticides registration action document: Sodium carbonate peroxyhydrate (PC Code 128860). USEPA Office of Pesticides Program, Biopesticides and Pollution Prevention Division.
Vodacek, A., Blough, N., De Grandpre, M. D., & Peltzer, E. (1999). Rapid and precise determination of dissolved oxygen by spectrophotometry: evaluation of interference from color and turbidity. Limnol Oceanogr, 44, 1148–1154.
Wetzel, R. G. (2001). Limnology: Lakes and river ecosystems (3rd ed.). San Diego: Academic Press.
Wolfe, W. C. (1962). Spectrophotometric determination of hydroperoxide in diethyl ether. Anal Chem, 34, 1328–1330.
Acknowledgments
The authors would like to thank Lonza Group for the funding support provided for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kinley, C.M., Rodgers, J.H., Iwinski, K.J. et al. Analysis of Algaecide Exposures: an Evaluation of the I3 − Method to Measure Sodium Carbonate Peroxyhydrate Algaecides. Water Air Soil Pollut 226, 170 (2015). https://doi.org/10.1007/s11270-015-2439-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2439-x