Abstract
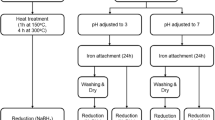
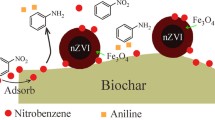
To obtain a good catalytic effect of removing refractory organics from water by Fenton process, granular activated carbon (GAC) supported nano-zero valent iron (nZVI) composite (nZVI/GAC) was prepared by adsorption–reduction method, and characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and energy-dispersive X-ray spectroscopy (EDS). The catalytic degradation activity of the composite was evaluated to remove nitrobenzene (NB) pollutant via a heterogeneous Fenton-like system, and the initial pH value, nZVI/GAC dosage, and H2O2 concentration influencing on NB removal were also investigated at room temperature. Experimental results showed that nZVI particle was uniformly dispersed over GAC matrix, and average particle size was 40–100 nm without agglomeration. The nZVI/GAC composite was very efficient in removing NB with average percentage of more than 85 %. However, the removal rate of Fenton-like reaction was highly affected by pH value, H2O2 concentration, and nZVI/GAC dosage. The optimal reaction conditions were pH 4.0, 40 mg/L NB, 5.0 mmol/L H2O2, and 0.4 g/L nZVI/GAC in this study. Stability and repeatability tests as well as mechanism analysis illustrated that GAC improved catalytic action via enhancing nZVI dispersion and accelerating Fe(III)/Fe(II) cycle attributing to internal iron–carbon microelectrolysis in nZVI/GAC composite. Iron utilization efficiency, which played an important role in NB degradation by Fenton-like greatly increased resulting in dissolved iron <0.6 mg/L. This phenomenon strongly implied that the nZVI/GAC Fenton-like process was not only a practical combination of adsorption and Fenton oxidation but also some synergetic effects existing in such an nZVI/GAC composite.








Similar content being viewed by others
References
Andreozzi, R., Caprio, V., Insola, A., & Marotta, R. (1999). Advanced oxidation processes (AOP) for water purification and recovery. Catalysis Today, 53(1), 51–59.
Bach, A., & Semiat, R. (2011). The role of activated carbon as a catalyst in GAC/iron oxide/H2O2 oxidation process. Desalination, 273(1), 57–63.
Bokare, A. D., & Choi, W. Y. (2014). Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. Journal of Hazardous Material, 275, 121–135.
Busch, J., Meißner, T., Potthoff, A., & Oswald, S. E. (2014). Transport of carbon colloid supported nanoscale zero-valent iron in saturated porous media. Journal of Contaminant Hydrology, 164, 25–34.
Carrasco-Marín, F., Mueden, A., & Moreno-Castilla, C. (1998). Surface-treated activated carbons as catalysts for the dehydration and dehydrogenation reactions of ethanol. Journal of Physical Chemistry B, 102, 9239–9244.
Chan, K. H., & Chu, W. (2003). The system design of atrazine oxidation by catalytic oxidation process through a kinetic approach. Water Research, 37(16), 3997–4003.
Chen, M., Cui, L., Li, C. H., & Diao, G. W. (2009a). Adsorption, desorption and condensation of nitrobenzene solution from active carbon: A comparison of two cyclodextrins and two surfactants. Journal of Hazardous Materials, 162(1), 23–28.
Chen, Q. Q., Wu, P. X., Li, Y. Y., Zhu, N. W., & Dang, Z. (2009b). Heterogeneous photo-Fenton photodegradation of reactive brilliant orange X-GN over iron-pillared montmorillonite under visible irradiation. Journal of Hazardous Materials, 168(2-3), 901–908.
Dou, X. M., Li, R., Zhao, B., & Liang, W. Y. (2010). Arsenate removal from water by zero-valent iron/activated carbon galvanic couples. Journal of Hazardous Materials, 182(1-3), 108–114.
Figueiredo, J. L., Pereira, M. F. R., Freitas, M. M. A., & Órfão, J. J. M. (1999). Modification of the surface chemistry of activated carbons. Carbon, 37(9), 1379–1389.
Ganzenko, O., Huguenot, D., van Hullebusch, E. D., Esposito, G., & Oturan, M. A. (2014). Electrochemical advanced oxidation and biological processes for wastewater treatment: A review of the combined approaches. Environmental Science and Pollution Research, 21(14), 8493–8524.
Ghatak, H. R. (2014). Advanced oxidation processes for the treatment of biorecalcitrant organics in wastewater. Critical Reviews in Environmental Science and Technology, 44(11), 1167–1219.
Gogate, P. R., & Pandit, A. B. (2004). A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Advances in Environmental Research, 8(3-4), 501–551.
Gu, Z. M., Fang, J., & Deng, B. L. (2005). Preparation and evaluation of GAC-based iron containing adsorbents for arsenic removal. Environmental Science and Technology, 39(10), 3833–3843.
Haigler, B. E., & Spain, J. C. (1991). Biotransformation of nitrobenzene by bacteria containing toluene degradative pathways. Applied and Environmental Microbiology, 57(11), 3156–3162.
Huang, L. H., Zhou, S. J., Jin, F., Huang, J., & Bao, N. (2014). Characterization and mechanism analysis of activated carbon fiber felt-stabilized nanoscale zero-valent iron for the removal of Cr (VI) from aqueous solution. Colloid and Surface A: Physicochemical and Engineering Aspects, 447, 59–66.
Hung, H. M., Ling, F. H., & Hoffmann, M. R. (2000). Kinetics and mechanism of the enhanced reductive degradation of nitrobenzene by elemental iron in the presence of ultrasound. Environmental Science & Technology, 34(9), 1758–1763.
Jadhav, A. J., & Srivastava, V. C. (2013). Adsorbed solution theory based modeling of binary adsorption of nitrobenzene, aniline and phenol onto granulated activated carbon. Chemical Engineering Journal, 229, 450–459.
Kato, Y., Machida, M., & Tatsumoto, H. (2008). Inhibition of nitrobenzene adsorption by water cluster formation at acidic oxygen functional groups on activated carbon. Journal of Colloid and Interface Science, 322(2), 394–398.
Kerkez, D. V., Tomašević, D. D., Kozma, G., Bečelić-Tomin, M. R., Prica, M. D., Ronćević, S. D., Kukovecz, A., Dalmacija, B. D., & Kónya, Z. (2014). Three different clay-supported nanoscale zero-valent iron materials for industrial azo dye degradation: A comparative study. Journal of Taiwan Institute of Chemical Engineers, 45(5), 2451–2461.
Kim, S. A., Kamala-Kannan, S., Lee, K. J., Park, Y. J., Shea, P. J., Lee, W. H., Kim, H. M., & Oh, B. T. (2013). Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chemical Engineering Journal, 217, 54–60.
Lam, S. W., Chiang, K., Lim, T. M., Amal, R., & Low, G. K. C. (2005). The role of ferric ion in the photochemical and photocatalytic oxidation of resorcinol. Journal of Catalysis, 234(2), 292–299.
Liang, B., Cheng, H. Y., Van Nostrand, J. D., Ma, J. C., Yu, H., Kong, D. Y., Liu, W. Z., Ren, N. Q., Wu, L. Y., Wang, A. J., Lee, D. J., & Zhou, J. Z. (2014). Microbial community structure and function of nitrobenzene reduction biocathode in response to carbon source switchover. Water Research, 54, 137–148.
Liao, Q., Sun, J., & Gao, L. (2009). Degradation of phenol by heterogeneous Fenton reaction using multi-walled carbon nanotube supported Fe2O3 catalysts. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 345(1-3), 95–100.
Mackenzie, K., Bleyl, S., Georgi, A., & Kopinke, F. D. (2012). Carbo-Iron-An Fe/AC composite-As alternative to nano-iron for groundwater treatment. Water Research, 46(12), 3817–3826.
Majumder, P. S., & Gupta, S. K. (2003). Hybrid reactor for priority pollutant nitrobenzene removal. Water Research, 37(18), 4331–4336.
Medien, H. A. A., & Khalil, S. M. E. (2010). Kinetics of the oxidative decolorization of some organic dyes utilizing Fenton-like reaction in water. Journal of King Saud University-Science, 22(3), 147–153.
Messele, S. A., Soares, O. S. G. P., Órfão, J. J. M., Bengoa, C., Stüber, F., Fortuny, A., Fabregat, A., & Font, J. (2015). Effect of activated carbon surface chemistry on the activity of ZVI/AC catalysts for Fenton-like oxidation of phenol. Catalysis Today, 240, 73–79.
Moreno-Castilla, C., Carrasco-Marin, F., Maldonado-Hodar, F. J., & Rivera-Utrilla, J. (1998). Effects of non-oxidant and oxidant acid treatments on the surface properties of an activated carbon with very low ash content. Carbon, 36(1-2), 145–151.
Mu, Y., Rozendal, R. A., Rabaey, K., & Keller, J. (2009). Nitrobenzene removal in bioelectrochemical systems. Environmental Science & Technology, 43(22), 8690–8695.
Nguyen, T. D., Phan, N. H., Do, M. H., & Ngo, K. T. (2011). Magnetic Fe2MO4 (M: Fe, Mn) activated carbons: Fabrication, characterization and heterogeneous Fenton oxidation of methyl orange. Journal of Hazardous Materials, 185(2-3), 653–661.
Nichela, D., Carlos, L., & Einschlag, F. G. (2008). Autocatalytic oxidation of nitrobenzene using hydrogen peroxide and Fe (III). Applied Catalysis B: Environmental, 82(1-2), 11–18.
Nurmi, J. T., Tratnyek, P. G., Sarathy, V., Baer, D. R., Amonette, J. E., Pecher, K., Wang, C. M., Linehan, J. C., Matson, D. W., Penn, R. L., & Driessen, M. D. (2005). Characterization and properties of metallic iron nanoparticles: spectroscopy, electrochemistry, and kinetics. Environmental Science & Technology, 39(5), 1221–1230.
Oturan, M. A., & Aaron, J. J. (2014). Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Critical Reviews in Environmental Science and Technology, 44(23), 2577–2641.
Parra, S., Nadtotechenko, V., Albers, P., & Kiwi, J. (2004). Discoloration of azo-dyes at biocompatible pH-values through an Fe-histidine complex immobilized on Nafion via Fenton-like processes. Journal of Physical Chemistry B, 108, 4439–4448.
Pereira, M. C., Coelho, F. S., Nascentes, C. C., Fabris, J. D., Araújo, M. H., Sapag, K., Luiz, C. A. O., & Lago, R. M. (2010). Use of activated carbon as a reactive support to produce highly active-regenerable Fe-based reduction system for environmental remediation. Chemosphere, 81(1), 7–12.
Pérez, M. C. M., Martínez, S., de Lecea, C., & Linares, S. A. (1997). Platinum supported on activated carbon cloths as catalyst for nitrobenzene hydrogenation. Applied Catalysis A: General, 151(2), 461–475.
Pignatello, J. J., Oliveros, E., & MacKay, A. (2006). Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Critical Reviews in Environmental Science and Technology, 36(1), 1–84.
Ramirez, J. H., Maldonado-Hódar, F. J., Pérez-Cadenas, A. F., Moreno-Castilla, C., Costa, C. A., & Madeira, L. M. (2007). Azo-dye Orange II degradation by heterogeneous Fenton like reaction using carbon-Fe catalysts. Applied Catalysis B: Environmental, 75(3-4), 312–323.
Romero, A., Santos, A., & Vicente, F. (2009). Chemical oxidation of 2, 4-dimethylphenol in soil by heterogeneous Fenton process. Journal of Hazardous Materials, 162(2-3), 785–790.
Segura, Y., Martínez, F., & Melero, J. A. (2013). Effective pharmaceutical wastewater degradation by Fenton oxidation with zero-valent iron. Applied Catalysis B: Environmental, 136–137, 64–69.
Shi, J. G., Ai, Z. H., & Zhang, L. Z. (2014). Fe@Fe2O3 core-shell nanowires enhanced Fenton oxidation by accelerating the Fe (III)/Fe (II) cycles. Water Research, 59, 145–153.
Singh, P., Raizada, P., Kumari, S., Kumar, A., Pathania, D., & Thakur, P. (2014). Solar-Fenton removal of malachite green with novel Fe0-activated carbon nanocomposite. Applied Catalysis A: Genenral, 476, 9–18.
Su, Y. F., Cheng, Y. L., & Shih, Y. H. (2013). Removal of trichloroethylene by zerovalent iron/activated carbon derived from agricultural wastes. Journal of Environmental Management, 129, 361–366.
Subbaramaiah, P. V., Srivastava, V. C., & Mall, I. D. (2014). Catalytic oxidation of nitrobenzene by copper loaded activated carbon. Separation and Purification Technology, 125, 284–290.
Tan, D., Zeng, H. H., Liu, J., Yu, X. Z., Liang, Y. P., & Lu, L. J. (2013). Degradation kinetics and mechanism of trace nitrobenzene by granular activated carbon enhanced microwave/hydrogen peroxide system. Journal of Environmental Science, 25(7), 1492–1499.
Thankappan, R., Nguyen, T. V., Srinivasan, S. V., Vigneswaran, S., Kandasamy, J., & Loganathan, P. (2015). Removal of leather tanning agent syntan from aqueous solution using Fenton oxidation followed by GAC adsorption. Journal of Industrial and Engineering Chemistry, 21, 483–488.
Velo-Gala, I., Lopez-Penalver, J. J., Sanchez-Polo, M., & Rivera-Utrilla, J. (2014). Comparative study of oxidative degradation of sodium diatrizoate in aqueous solution by H2O2/Fe2+, H2O2/Fe3+, Fe (VI) and UV, H2O2/UV, K2S2O8/UV. Chemical Engineering Journal, 241, 504–512.
Wang, W., Zhou, M., Mao, Q., Yue, J., & Wang, X. (2010). Novel NaY zeolite-supported nanoscale zero-valent iron as an efficient heterogeneous Fenton catalyst. Catalysis Communications, 11(11), 937–941.
Wang, A. J., Cheng, H. Y., Liang, B., Ren, N. Q., Cui, D., Lin, N., Kim, B. H., & Rabaey, K. (2011). Efficient reduction of nitrobenzene to aniline with a biocatalyzed cathode. Environmental Science & Technology, 45(23), 10186–10193.
Wang, L., Yao, Y. Y., Zhang, Z. H., Sun, L. J., Lu, W. Y., Chen, W. X., & Chen, H. X. (2014). Activated carbon fibers as an excellent partner of Fenton catalyst for dyes decolorization by combination of adsorption and oxidation. Chemical Engineering Journal, 251, 348–354.
Wu, J. H., Yin, W. Z., Gu, J. J., Li, P., Wang, X. D., & Yang, B. (2013a). A biotic Fe0-H2O system for nitrobenzene removal from groundwater. Chemical Engineering Journal, 226, 14–21.
Wu, L. M., Liao, L. B., Lv, G. C., Qin, F. X., He, Y. J., & Wang, X. Y. (2013b). Micro-electrolysis of Cr(VI) in the nanoscale zero-valent iron loaded activated carbon. Journal of Hazardous Materials, 254–255, 277–283.
Wu, S. C., Wen, G. D., Zhong, B. W., Zhang, B. S., Gu, X. M., Wang, N., & Su, D. S. (2014). Reduction of nitrobenzene catalyzed by carbon materials. Chinese Journal of Catalysis, 35(6), 914–921.
Xiao, J. N., Yue, Q. Y., Gao, B. Y., Sun, Y. Y., Kong, J. J., Gao, Y., Li, Q., & Wang, Y. (2014). Performance of activated carbon/nanoscale zero-valent iron for removal of trihalomethanes (THMs) at infinitesimal concentration in drinking water. Chemical Engineering Journal, 23, 63–72.
Yao, Y. Y., Wang, L., Sun, L. J., Zhu, S., Huang, Z. F., Mao, Y. J., Lu, W. Y., & Chen, W. X. (2013). Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chemical Engineering Science, 101, 424–431.
Zhang, H., Jin, Z., Han, L., & Qin, C. (2006). Synthesis of nanoscale zero valent iron supported on exfoliated graphite for removal of nitrate. Transactions of Nonferrous Metals Society of China, 16(1), 345–349.
Zhang, R. M., Li, J. S., Liu, C., Shen, J. Y., Sun, X. Y., Han, W. Q., & Wang, L. J. (2013a). Reduction of nitrobenzene using nanoscale zero-valent iron confined in channels of ordered mesoporous silica. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 425, 108–114.
Zhang, Y. Y., Jiang, H., Zhang, Y., & Xie, J. F. (2013b). The dispersity-dependent interaction between montmorillonite supported nZVI and Cr(VI) in aqueous solution. Chemical Engineering Journal, 229, 412–419.
Zhang, Y. L., Zhang, K., Dai, C. M., & Zhou, X. F. (2014). Performance and mechanism of pyrite for nitrobenzene removal in aqueous solution. Chemical Engineering Science, 111, 135–141.
Zhou, H. M., Shen, Y. Y., Lv, P., Wang, J. J., & Fan, J. (2013). Degradation of 1-butyl-3-methylimidazolium chloride ionic liquid by ultrasound and zero-valent iron/activated carbon. Separation and Purification Technology, 104, 208–213.
Acknowledgments
The authors gratefully acknowledge the financial support by the National Natural Science Foundation of China (Grant No. 40872164), fund for basic research from the Northwestern Polytechnical University (No. JCY20130145), and China geological survey project (12120114056201).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, S., Yao, H., Wang, K. et al. Intensify Removal of Nitrobenzene from Aqueous Solution Using Nano-Zero Valent Iron/Granular Activated Carbon Composite as Fenton-Like Catalyst. Water Air Soil Pollut 226, 155 (2015). https://doi.org/10.1007/s11270-015-2421-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2421-7