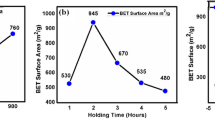
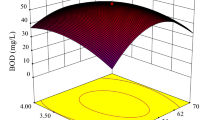
Abstract
We need specific and competent adsorbents to remove arsenic and bring it down to permissible levels in drinking water. Therefore, industrial byproducts are extensively applied to produce large amounts of natural adsorbents. Similarly, managing optimum arsenic adsorption with palm oil clinker sand (POCS) is possible through a careful statistical planning of adsorption variables. We plan and perform a minimum number of experiments to (1) obtain optimum arsenic adsorption and (2) provide a new possible application opportunity to the industrial waste managers and future planners. We observed that adsorption of arsenic was dependent on the pH of the system, initial concentration of arsenic (mg L−1), amount (mg) of POCS, and temperature of the bio-adsorption system. A correlation among the study variables was constructed by three-dimensional (3D) response surfaces and two-dimensional (2D) contour plots based on central composite design (CCD) experiments in a batch mode of study. A quadratic model fitted well with the experimental data and better explained the superiority of current bio-adsorption system and efficient removal of arsenic from water samples. We confirmed that the selected variables were experimentally and statistically significant and controlled the overall adsorption response by the batch system. A comparative and thorough analysis of the adsorption process confirmed that selected variables were mutually interacting in a nonlinear fashion in this study. Excellent experimental results and external comparative studies prove the relative importance of the present model and adsorption system for arsenic remediation biotechnology.






Similar content being viewed by others

References
Aghaeinejad-Meybodi, A., Ebadi, A., Shafiei, S., Khataee, A. R., & Rostampour, M. (2015). Modeling and optimization of antidepressant drug fluoxetine removal in aqueous media by ozone/H2O2 process: comparison of central composite design and artificial neural network approaches. J Taiwan Inst Chem Eng, 48(0), 40–48. doi:10.1016/j.jtice.2014.10.022.
Ahmad, T., Rafatullah, M., Ghazali, A., Sulaiman, O., & Hashim, R. (2011). Oil palm biomass-based adsorbents for the removal of water pollutants—a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev, 29(3), 177–222. doi:10.1080/10590501.2011.601847.
Ahmmad, R., Jumaat, M. Z., Bahri, S., & Islam, A. B. M. S. (2014). Ductility performance of lightweight concrete element containing massive palm shell clinker. Construct Build Mater, 63, 234–241. doi:10.1016/j.conbuildmat.2014.04.022.
Anirudhan, T. S., Divya, L., & Parvathy, J. (2013). Arsenic adsorption from contaminated water on Fe (III)-coordinated amino-functionalized poly(glycidylmethacrylate)-grafted TiO2-densified cellulose. J Chem Technol Biotechnol, 88(5), 878–886. doi:10.1002/jctb.3916.
Arcibar-Orozco, J. A., Josue, D.-B., Rios-Hurtado, J. C., & Rangel-Mendez, J. R. (2014). Influence of iron content, surface area and charge distribution in the arsenic removal by activated carbons. Chem Eng J, 249, 201–209. doi:10.1016/j.cej.2014.03.096.
Badkar, D., Pandey, K., & Buvanashekaran, G. (2013). Development of RSM- and ANN-based models to predict and analyze the effects of process parameters of laser-hardened commercially pure titanium on heat input and tensile strength. Int J Adv Manuf Technol, 65(9–12), 1319–1338. doi:10.1007/s00170-012-4259-0.
Bailey, S. E., Olin, T. J., Bricka, R. M., & Adrian, D. D. (1999). A review of potentially low-cost sorbents for heavy metals. Water Res, 33(11), 2469–2479. doi:10.1016/s0043-1354(98)00475-8.
Bibi, S., Farooqi, A., Hussain, K., & Haider, N. (2015). Evaluation of industrial based adsorbents for simultaneous removal of arsenic and fluoride from drinking water. J Clean Prod, 87, 882–896. doi:10.1016/j.jclepro.2014.09.030.
Bilici Baskan, M., & Pala, A. (2010). A statistical experiment design approach for arsenic removal by coagulation process using aluminum sulfate. Desalination, 254(1–3), 42–48. doi:10.1016/j.desal.2009.12.016.
Dias, J. M., Alvim-Ferraz, M. C. M., Almeida, M. F., Rivera-Utrilla, J., & Sanchez-Polo, M. (2007). Waste materials for activated carbon preparation and its use in aqueous-phase treatment: a review. J Environ Manage, 85(4), 833–846. doi:10.1016/j.jenvman.2007.07.031.
Dixit, S., & Hering, J. G. (2003). Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Tech, 37(18), 4182–4189. doi:10.1021/es030309t.
Dora, T. K., Mohanty, Y. K., Roy, G. K., & Sarangi, B. (2013). Adsorption studies of As (III) from wastewater with a novel adsorbent in a three-phase fluidized bed by using response surface method. J Environ Chem Eng, 1(3), 150–158. doi:10.1016/j.jece.2013.04.011.
Ferraro, J.R. (2012) Practical Fourier transform infrared spectroscopy: industrial and laboratory chemical analysis. Elsevier.
Hameed, B. H., Tan, I. A., & Ahmad, A. L. (2009). Preparation of oil palm empty fruit bunch-based activated carbon for removal of 2,4,6-trichlorophenol: optimization using response surface methodology. J Hazard Mater, 164(2–3), 1316–1324. doi:10.1016/j.jhazmat.2008.09.042.
Han, C., Pu, H., Li, H., Deng, L., Huang, S., He, S., & Luo, Y. (2013). The optimization of As (V) removal over mesoporous alumina by using response surface methodology and adsorption mechanism. J Hazard Mater, 254–255, 301–309. doi:10.1016/j.jhazmat.2013.04.008.
Kim, C. S., Chi, C., Miller, S. R., Rosales, R. A., Sugihara, E. S., Akau, J., Rytuba, J. J., & Webb, S. M. (2013). (Micro) spectroscopic analyses of particle size dependence on arsenic distribution and speciation in mine wastes. Environ Sci Tech, 47, 8164–8171. doi:10.1021/es4010653.
Li, J., Lu, H., Guo, J., Xu, Z. M., & Zhou, Y. H. (2007). Recycle technology for recovering resources and products from waste printed circuit boards. Environ Sci Tech, 41(6), 1995–2000. doi:10.1021/es0618245.
Naseri, E., Reyhanitabar, A., Oustan, S., Heydari, A. A., & Alidokht, L. (2014). Optimization arsenic immobilization in a sandy loam soil using iron-based amendments by response surface methodology. Geoderma, 232–234, 547–555. doi:10.1016/j.geoderma.2014.06.009.
Nieto-Delgado, C., & Rangel-Mendez, J. R. (2012). Anchorage of iron hydro (oxide) nanoparticles onto activated carbon to remove As (V) from water. Water Res, 46(9), 2973–2982. doi:10.1016/j.watres.2012.03.026.
Oh, S.-Y., & Yoon, M.-K. (2013). Chemical extraction of arsenic from contaminated soils in the vicinity of abandoned mines and a smelting plant under subcritical conditions. Soil Sediment Contam, 23(2), 166–179. doi:10.1080/15320383.2014.808169.
Roig, A., Cayuela, M. L., & Sanchez-Monedero, M. A. (2006). An overview on olive mill wastes and their valorisation methods. Waste Manag, 26(9), 960–969. doi:10.1016/j.wasman.2005.07.024.
Roy, P., Mondal, N. K., & Das, K. (2014). Modeling of the adsorptive removal of arsenic: a statistical approach. J Environ Chem Eng, 2(1), 585–597. doi:10.1016/j.jece.2013.10.014.
Ryan, J. V., Lemieux, P. M., Pollard, K., Workman, R., Antley, B., & Yurk, J. (2000). Characterization of organic emissions from hazardous waste incineration processes under the new EPA draft Risk Burn Guidance: measurement issues. Waste Manag, 20(5–6), 347–353. doi:10.1016/s0956-053x (99)00336-0.
Sen Gupta, B., Chatterjee, S., Rott, U., Kauffman, H., Bandopadhyay, A., DeGroot, W., Nag, N. K., Carbonell-Barrachina, A. A., & Mukherjee, S. (2009). A simple chemical free arsenic removal method for community water supply—a case study from West Bengal, India. Environ Pollut, 157(12), 3351–3353. doi:10.1016/j.envpol.2009.09.014.
Shanmugaprakash, M., & Sivakumar, V. (2013). Development of experimental design approach and ANN-based models for determination of Cr (VI) ions uptake rate from aqueous solution onto the solid biodiesel waste residue. Bioresour Technol, 148, 550–559. doi:10.1016/j.biortech.2013.08.149.
Simsek, E. B., Özdemir, E., & Beker, U. (2013). Process optimization for arsenic adsorption onto natural zeolite incorporating metal oxides by response surface methodology. Water Air Soil Pollut, 224(7), 1614. doi:10.1007/s11270-013-1614-1.
Singh, R., Singh, S., Parihar, P., Singh, V. P., & Prasad, S. M. (2015). Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf, 112, 247–270. doi:10.1016/j.ecoenv.2014.10.009.
Smith, A. H., Lingas, E. O., & Rahman, M. (2000). Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ, 78(9), 1093–1103.
Trois, C., & Cibati, A. (2015). South African sands as a low cost alternative solution for arsenic removal from industrial effluents in permeable reactive barriers: column tests. Chem Eng J, 259, 981–989. doi:10.1016/j.cej.2014.08.063.
Tuna, A. Ö. A., Özdemir, E., Simsek, E. B., & Beker, U. (2013). Optimization of process parameters for removal of arsenic using activated carbon-based iron-containing adsorbents by response surface methodology. Water Air Soil Pollut, 224(9), 1685. doi:10.1007/s11270-013-1685-z.
Wang, Y., & Jiao, J. J. (2014). Multivariate statistical analyses on the enrichment of arsenic with different oxidation states in the Quaternary sediments of the Pearl River Delta, China. J Geochem Explor, 138, 72–80. doi:10.1016/j.gexplo.2013.12.012.
Witek-Krowiak, A., Chojnacka, K., Podstawczyk, D., Dawiec, A., & Pokomeda, K. (2014). Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour Technol, 160, 150–160. doi:10.1016/j.biortech.2014.01.021.
Zaman, A. U., & Lehmann, S. (2013). The zero waste index: a performance measurement tool for waste management systems in a ‘zero waste city’. J Clean Prod, 50, 123–132. doi:10.1016/j.jclepro.2012.11.041.
Acknowledgments
The authors gratefully acknowledge Bright Spark Program, University of Malaya and High Impact Research MoE Grant UM.C/625/1/HIR/MoE/SC/04/01 from the Ministry of Education Malaysia and Centre for Ionic Liquids (UMCiL) for the support/assistance provided to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, M.A., Yusoff, I., Ahmmad, R. et al. Arsenic Adsorption Using Palm Oil Waste Clinker Sand Biotechnology: an Experimental and Optimization Approach. Water Air Soil Pollut 226, 149 (2015). https://doi.org/10.1007/s11270-015-2411-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2411-9