Abstract
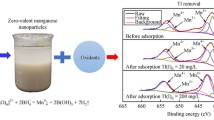
The adsorption and oxidation of thallium(I) by nanosized manganese dioxide (nMnO2) may have an impact on the removal of Tl from waters in engineered applications, as well as the fate and transport of Tl in natural waters. The fundamental data on the adsorption and oxidation of Tl(I) by nMnO2 were obtained here. The results show that Tl was adsorbed by nMnO2 within 15 min at pH 7.0. Moreover, Langmuir fitting indicated a maximum adsorption capacity of ∼58.48 mg/mmol (i.e., ∼672 mgTl/gMnO2). The presence of Ca2+, Mg2+, SiO3 2−, PO4 3−, and CO3 2−decreased the removal of Tl(I) to a certain extent; however, it was increased by a pH from 4.0 to 9.0. The oxidation of Tl(I) was proposed at pH 4.0 based on the observation of Mn release and nMnO2 aggregation, while the oxidation of Tl(I) might not be favored at neutral and basic conditions. The presence of 3 mg/L humic acid hindered the adsorption of Tl(I) on nMnO2. These results indicate that nMnO2 could help to remove Tl from water in engineered applications and might deepen our understanding of the transport of Tl in natural waters.







Similar content being viewed by others
References
Baker, R. G. A., Rehkamper, M., Hinkley, T. K., Nielsen, S. G., & Toutain, J. P. (2009). Investigation of thallium fluxes from subaerial volcanism-implications for the present and past mass balance of thallium in the oceans. Geochim. Cosmochim. Acta, 73, 6340–6359.
Buffle, J., & Leppard, G. G. (1995). Characterization of aquatic colloids and macromolecules. 1. Structure and behavior of colloidal material. Environmental Science & Technology, 29, 2169–2175.
Chen, K. L., & Elimelech, M. (2006). Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir, 22, 10994–11001.
Cvjetko, P., Cvjetko, I., & Pavlica, M. (2010). Thallium toxicity in humans. Arhiv Za Higijenu Rada I Toksikologiju, 61, 111–119.
Ferreira, J. R., Lawlor, A. J., Bates, J. M., Clarke, K. J., & Tipping, E. (1997). Chemistry of riverine and estuarine suspended particles from the Ouse-Trent system, UK. Colloids Surf. A Physicochem. Eng. Asp, 120, 183–198.
Fu, G. M., Allen, H. E., & Cowan, C. E. (1991). Adsorption of cadmium and copper by manganese oxide. Soil Science, 152, 72–81.
Galvan-Arzate, S., & Santamaria, A. (1998). Thallium toxicity. Toxicology Letters, 99, 1–13.
Herszage, J., dos Santos Afonso, M., & Luther, G. W. (2003). Oxidation of cysteine and glutathione by soluble polymeric MnO2. Environmental Science & Technology, 37, 3332–3338.
Hua, M., Zhang, S., Pan, B., Zhang, W., Lv, L., & Zhang, Q. (2012). Heavy metal removal from water/wastewater by nanosized metal oxides: a review. Journal of Hazardous Materials, 211–212, 317–331.
Huangfu, X., Jiang, J., Ma, J., Liu, Y., & Yang, J. (2013). Aggregation kinetics of manganese dioxide colloids in aqueous solution: influence of humic substances and biomacromolecules. Environmental Science & Technology, 47, 10285–10292.
Jiang, J., Pang, S. Y., & Ma, J. (2009). Oxidation of triclosan by permanganate (Mn(VII)): importance of ligands and in situ formed manganese oxides. Environmental Science & Technology, 43, 8326–8331.
Johnson, B. B. (1990). Effect of Ph, temperature, and concentration on the adsorption of cadmium on goethite. Environmental Science & Technology, 24, 112–118.
Lafferty, B. J., Ginder-Vogel, M., Zhu, M., Livi, K. J., & Sparks, D. L. (2010). Arsenite oxidation by a poorly crystalline manganese-oxide. 2. Results from X-ray absorption spectroscopy and X-ray diffraction. Environmental Science & Technology, 44, 8467–8472.
Landrot, G., Ginder-Vogel, M., Livi, K., Fitts, J. P., & Sparks, D. L. (2012). Chromium(III) oxidation by three poorly-crystalline manganese(IV) oxides. 1. Chromium(III)-oxidizing capacity. Environmental Science & Technology, 46, 11594–11600.
Law, S., & Turner, A. (2011). Thallium in the hydrosphere of south west England. Environmental Pollution, 159, 3484–3489.
Lienemann, C. P., Taillefert, M., Perret, D., & Gaillard, J. F. (1997). Association of cobalt and manganese in aquatic systems: chemical and microscopic evidence. Geochim. Cosmochim. Acta, 61, 1437–1446.
Lin, T. S., & Nriagu, J. (1998). Revised hydrolysis constants for thallium(I) and thallium(III) and the environmental implications. Journal of the Air & Waste Management Association, 48, 151–156.
Lin, T. S., & Nriagu, J. (1999). Thallium speciation in the Great Lakes. Environmental Science & Technology, 33, 3394–3397.
Liu, J., Lippold, H., Wang, J., Lippmann-Pipke, J., & Chen, Y. (2011). Sorption of thallium(I) onto geological materials: influence of pH and humic matter. Chemosphere, 82, 866–871.
Lockwood, R. A., & Chen, K. Y. (1973). Adsorption of Hg(ll) by hydrous manganese oxides. Environmental Science & Technology, 7, 1028–1034.
Ma, J., & Graham, N. (1996). Controlling the formation of chloroform by permanganate preoxidation—destruction of precursors. J. Water Supply Res. Technol. AQUA, 45, 308–315.
Ma, J., Jiang, J., Pang, S., & Guo, J. (2007). Adsorptive fractionation of humic acid at air-water interfaces. Environmental Science & Technology, 41, 4959–4964.
Mathis, B. J., & Kevern, N. R. (1975). Distribution of mercury, cadmium, lead, and thallium in a eutrophic lake. Hydrobiologia, 46, 207–222.
Matthews, A. D., & Riley, J. P. (1970). The occurrence of thallium in sea water and marine sediments. Chemical Geology, 6, 149–152.
Memon, S. Q., Memon, N., Solangi, A. R., & Memon, J. U. R. (2008). Sawdust: a green and economical sorbent for thallium removal. Chemical Engineering Journal, 140, 235–240.
Murray, J. W. (1974a). The interaction of metal ions at the manganese dioxide-solution interface. Geochim. Cosmochim. Acta, 39, 505–519.
Murray, J. W. (1974b). The surface chemistry of hydrous manganese dioxide. Journal of Colloid and Interface Science, 46, 357–371.
Nielsen, S. G., Mar-Gerrison, S., Gannoun, A., LaRowe, D., Klemm, V., Halliday, A. N., Burton, K. W., & Hein, J. R. (2009). Thallium isotope evidence for a permanent increase in marine organic carbon export in the early Eocene. Earth and Planetary Science Letters, 278, 297–307.
Peacock, C. L., & Moon, E. M. (2012). Oxidative scavenging of thallium by birnessite: explanation for thallium enrichment and stable isotope fractionation in marine ferromanganese precipitates. Geochim. Cosmochim. Acta, 84, 297–313.
Posselt, H. S., Anderson, F. J., & Walter, W. J. (1968). Cation sorption on colloidal hydrous manganese dioxide. Environmental Science & Technology, 2, 1087–1093.
Pua, Y., Yang, X., Zheng, H., Wang, D., Su, Y., & He, J. (2013). Adsorption and desorption of thallium (I) on multiwalled carbon nanotubes. Chemical Engineering Journal, 219, 403–410.
Qin, Q. D., Wang, Q. Q., Fu, D. F., & Ma, J. (2011). An efficient approach for Pb(II) and Cd(II) removal using manganese dioxide formed in situ. Chemical Engineering Journal, 172, 68–74.
Rehkämper, M., & Nielsen, S. G. (2004). The mass balance of dissolved thallium in the oceans. Marine Chemistry, 85, 125–139.
Rehkämper, M., Frank, M., Hein, J. R., & Halliday, A. (2004). Cenozoic marine geochemistry of thallium deduces from isotopic studies of ferromanganese crusts and pelagic sediments. Earth and Planetary Science Letters, 219, 77–91.
Senol, Z. M., & Ulusoy, U. (2010). Thallium adsorption onto polyacryamide-aluminosilicate composites: a Tl isotope tracer study. Chemical Engineering Journal, 162, 97–105.
Stone, A. T., & Morgan, J. J. (1984). Reduction and dissolution of manganese(III) and manganese(IV) oxides by organics. 1. Reaction with hydroquinone. Environmental Science & Technology, 18, 450–456.
Tripathy, S. S., Bersillon, J. L., & Gopal, K. (2006). Adsorption of Cd2+ on hydrous manganese dioxide from aqueous solutions. Desalination, 194, 11–21.
Twining, B. S., Twiss, M. R., & Fisher, N. S. (2003). Oxidation of thallium by freshwater plankton communities. Environmental Science & Technology, 37, 2720–2726.
Waite, T. D., Wrigley, I. C., & Szymczak, R. (1988). Photoassisted dissolution of a colloidal manganese oxide in the presence of fulvic acid. Environmental Science & Technology, 22, 778–785.
Wang, Z. M., Lee, S. W., Kapoor, P., Tebo, B. M., & Giammar, D. E. (2013). Uraninite oxidation and dissolution induced by manganese oxide: a redox reaction between two insoluble minerals. Geochim. Cosmochim. Acta, 100, 24–40.
Wigginton, N. S., Haus, K. L., & Hochella, M. F., Jr. (2007). Aquatic environmental nanoparticles. Journal of the Air & Waste Management Association, 9, 1306–1316.
Xiao, T., Yang, F., Li, S., Zheng, B., & Ning, Z. (2012). Thallium pollution in China: a geoenvironmental perspective. Science of the Total Environment, 421–422, 51–58.
Yao, W. S., & Millero, F. J. (1996). Adsorption of phosphate on manganese dioxide in seawater. Environmental Science & Technology, 30, 536–541.
Zitko, V. (1975). Toxicity and pollution potential of thallium. Science of the Total Environment, 4, 185–192.
Acknowledgments
This work was financially supported by the National Science and Technology Pillar Program, China (No. 2012BAC05B02), the Funds for Creative Research Groups of China (51121062), the National Natural Science Foundation of China (51008104), the funds of the State Key Laboratory of Urban Water Resource and Environment (HIT, 2013TS04), the Foundation for the Author of National Excellent Doctoral Dissertation of China (201346), and the Chinese Postdoctoral Science Foundation and the Special Financial Grant (20110490106 and 2012T50365).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huangfu, X., Jiang, J., Lu, X. et al. Adsorption and Oxidation of Thallium(I) by a Nanosized Manganese Dioxide. Water Air Soil Pollut 226, 2272 (2015). https://doi.org/10.1007/s11270-014-2272-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2272-7