Abstract
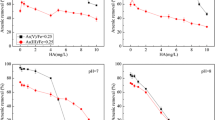
The effect of humic acid (HA), on the adsorption and transport of arsenic (As) onto and within a model iron oxide-based adsorbent, iron oxide-coated diatomite (IOCD), is investigated. Experimental results indicate that the adsorption of both As and HA is highly pH-dependent. As uptake was suppressed by HA, with the level of suppression increasing with HA concentration. The suppression is attributed to the partial coverage of the adsorption sites, as confirmed by elemental analysis. Adsorption energy analysis indicates that for As(III), the main interaction with IOCD is physical adsorption, whereas for As(V), it is more likely ion exchange. The presence of HA may alter the adsorption energy and interaction of As with the adsorbent, particularly at higher HA concentrations. Kinetic results indicate that HA did not affect the diffusional transport of As in systems with both As and HA. However, for IOCD preloaded with HA, the adsorption kinetics of As was significantly slower, although the As uptake was similar to the conditions of co-sorption with HA. The slower kinetics and similar equilibrium uptake of As in the HA-preloaded IOCD system may be attributed to the partial blockage of the intraparticular pores within IOCD, which slowed down the diffusion of As.





Similar content being viewed by others
References
Anawar, H. M., Akai, J., Komaki, K., Hiroshi, T., Takahito, Y., Toshio, I., Syed, S., & Kikuo, K. (2003). Geochemical occurrence of arsenic in groundwater of Bangladesh: source and mobilization processes. Journal of Geochemical Exploration, 77, 109–131.
Bahar, M. M., Megharaj, M., & Naidu, R. (2013). Bioremediation of As-contaminated water: recent advances and future prospects. Water, Air, and Soil Pollution, 224, 1722.
Baohua, G. U., Schmitt, J., Chen, Z., Liang, L., & McCarthy, J. F. (1994). Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environmental Science and Technology, 28(1), 38–46.
Bard, A. J., Parsons, R., & Jordan, J. (1985). Standard potentials in aqueous solution. New York: Dekker.
Bauer, M., & Blodau, C. (2006). Mobilization of As by dissolved organic matter from iron oxides, soils and sediments. Science of the Total Environment, 354, 179–190.
Benjamin, M. M., Sletten, R. S., Bailey, R. P., & Bennet, T. (1996). Sorption and filtration of metals using iron-oxide-coated sand. Water Research, 30(11), 2609–2619.
Bhunia, P., Pal, P., & Bandyopadhyay, M. (2007). Assessing As leachability from pulverized cement concrete produced from As-laden solid CalSiCo-sludge. Journal Hazardous Material, 141(3), 826–833.
Biswas, B.K., & Loeppert, R.H. (2003). Adsorption of As(V)/As(III) on TiO2 and the photocatalytic oxidation of As(III). 7th International Conference on the Biogeochemistry of Trace Elements, Uppsala, Sweden
Chabani, M., Amrane, A., & Bensmaili, A. (2009). Equilibrium sorption isotherms for nitrate on resin Amberlite IRA 400. J Hazard Mat, 165, 27–33.
Cullen, W. R., & Reimer, K. J. (1989). Arsenic speciation in the environment. Chemical Reviews, 89, 713–764.
Deschamps, E., Ciminelli, V., Weidler, P. G., & Ramos, A. Y. (2003). As sorption onto soils enriched in Mn and Fe minerals. Clays and Clay Minerals, 51, 197–204.
Dhiman, A. K., & Chaudhuri, M. (2007). Iron and manganese amended activated alumina—a medium for adsorption/oxidation of arsenic from water. Journal of Water Supply: Research and Technology - AQUA, 56, 69–74.
Dixit, S., & Hering, J. G. (2003). Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environmental Science and Technology, 37, 4182–4189.
Dubinin, M. M. (1960). The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chemical Reviews, 60, 235–266.
Eaton, A. (1995). Measuring UV-absorbing organics: a standard method. Journal AWWA, 87, 86–90.
Ferguson, J.F., & Anderson, M.A. (1974). Chemical forms of As in water supplies and their removal. In: Chemistry of water supply, treatment, and distribution; Rubin, A. J., Ed.; Ann Arbor Science, Ann Arbor, MI, 1974; pp 137–158.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156, 2–10.
Fox, L. E. (1983). The removal of dissolved humic acid during estuarine mixing. Estuarine, Coastal and Shelf Science, 16, 431–440.
Freundlich, H. M. F. (1906). Über die Adsorption in Lösungen. Zeitschrift für Physikalische Chemie-Leipzig, 57A, 385–470.
Giasuddin, A. B. M., Kanel, S. R., & Choi, H. (2007). Adsorption of humic acid onto nano scale zerovalent iron and its effect on As removal. Environmental Science and Technology, 41, 2022–2027.
Gu, B., Schmitt, J., Chen, Z., Liang, L., & McCarthy, J. F. (1994). Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environmental Science and Technology, 28, 38–46.
Guan, X., Dong, H., Ma, J., & Jiang, L. (2009). Removal of As from water: effects of competing anions on As(III) removal in KMnO4–Fe(II) process. Water Research, 43, 3891–3899.
Guo, H., Zhong, Z., Lei, M., Xue, X., Wan, X., Zhao, J., & Chen, T. (2012). As uptake from As-contaminated water using hyperaccumulator Pteris vittata L.: effect of chloride, bicarbonate, and As species. Water, Air, and Soil Pollution, 223, 4209–4220.
Harriott, P. (1962). Mass transfer to particles: part I. Suspended in agitated tanks. AIChE Journal, 8, 93–101.
Hasany, S. M., & Chaudhary, M. H. (1996). Sorption potential of Hare River sand for the removal of antimony from acidic aqueous solution. Applied Radiation and Isotopes, 47, 467–471.
Ho, Y. S., Huang, C. T., & Huang, H. W. (2002). Equilibrium sorption isotherm for metal ions on tree fern. Process Biochemistry, 37, 1421–1430.
Hristovski, K. D., Westerhoff, P. K., Möllerc, T., & Sylvester, P. (2009). Effect of synthesis conditions on nano-iron (hydr)oxide impregnated granulated activated carbon. Chemical Engineering Journal, 146, 237–243.
Jang, M., Chen, W., & Cannon, F. S. (2008). Preloading hydrous ferric oxide into granular activated carbon for arsenic removal. Environmental Science and Technology, 42, 3369–3374.
Jansen, S. A., Malaty, M., Nwabara, S., Johnson, E., Ghabbour, E., Davies, G., & Varnum, J. M. (1996). Structural modeling in humic acids. Materials Science and Engineering C, 4, 175–179.
Jeon, C. S., Baek, K., Park, J. K., Oh, Y. K., & Lee, S. D. (2009). Adsorption characteristics of AsV on iron-coated zeolite. Journal of Hazardous Materials, 163, 804–808.
Jiménez-Cedillo, M. J., Olguín, M. T., & Fall, C. (2009). Adsorption kinetic of arsenates as water pollutant on iron, manganese and iron-manganese-modified clinoptilolite-rich tuffs. Journal of Hazardous Materials, 163, 939–945.
Kalbitz, K., & Wennrich, R. (1998). Mobilization of heavy metals and arsenic in polluted wetland soils and its dependence on dissolved organic matter. Science of the Total Environment, 209, 27–39.
Kim, Y., Kim, C., Choi, I., Rengaraj, S., & YI, A. (2004). As removal using mesoporous alumina prepared via a templating method. Environmental Science and Technology, 38, 924–931.
Kundu, S., & Gupta, A. K. (2006). Arsenic adsorption onto iron oxide coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization. Chemical Engineering Journal, 122, 93–106.
Lai, C. H., Lo, S. L., & Chiang, H. L. (2000). Adsorption/desorption properties of copper ions on the surface of iron-coated sand using BET and EDAX analysis. Chemosphere, 41, 1249–1255.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361–1403.
Lin, T. F. (1997). Diffusion and sorption of water vapor and benzene within a dry model soil organic matter. Water Science and Technology, 35, 131–138.
Lin, T. F., & Wu, J. K. (2001). Adsorption of arsenite and arsenate within activated alumina grains: equilibrium and kinetics. Water Research, 35, 2049–2057.
Lin, T. F., Little, J. C., & Nazaroff, W. W. (1996). Transport and sorption of organic gases in activated carbon. Journal of Environmental Engineering, 122, 169–175.
Lin, T. F., Hsiao, H. C., Wu, J. K., Hsiao, H. C., & Yeh, J.-H. (2002). Removal of arsenic from groundwater using point-of-use reverse osmosis and distilling devices. Environmental Technology, 23, 781–79.
Lin, T. F., Liu, C. C., & Hsieh, W. H. (2006). Adsorption kinetics and equilibrium of arsenic onto an iron-based adsorbent and an ion exchange resin. Water Supply, 6(2), 201–207.
Liu, C., Wang, X., Li, X., Cao, W., & Yang, J. (2012). Detoxification of arsenite through adsorption and oxidative transformation on pyrolusite. Clean – Soil, Air, Water, 0, 1–8.
Liu, F., Fan, J., Wanga, S., & Mab, G. (2013). Adsorption of natural organic matter analogues by multi-walled carbon nanotubes: comparison with powdered activated carbon. Chemical Engineering Journal, 219, 450–458.
Loffredo, L., & Senesi, N. (2006). The role of humic substances in the fate of anthropogenic organic pollutants in soil with emphasis on endocrine disruptor compounds. I. Twardowska et al. (eds.), Soil and Water Pollution Monitoring, Protection and Remediation, 3–23.
Luo, L., Zhang, S., Shan, X. Q., & Zhu, Y. G. (2006). Effects of oxalate and humic acid on arsenate sorption by and desorption from a Chinese red soil. Water, Air, and Soil Pollution, 176, 269–283.
Lv, X., Hu, Y., Tang, J., Sheng, T., Jiang, G., & Xu, X. (2013). Effects of co-existing ions and natural organic matter on removal of chromium (VI) from aqueous solution by nanoscale zero valent iron (nZVI)-Fe3O4 nanocomposites. Chemical Engineering Journal, 218, 55–64.
Mandal, B. K., & Suzuki, K. T. (2002). Arsenic round the world: a review. Talanta, 58, 201–235.
Manna, B. R., Dey, S., Debnath, S., & Ghosh, U. C. (2003). Removal of As from groundwater using crystalline hydrous ferric oxide (CHFO). Water Quality Research Journal of Canada, 38(1), 193–210.
Markovski, J. S., Markovic, D. D., Dokic, V. R., Mitric, M., Ristic, M. D., Onjia, A. E., & Marinkovic, A. D. (2014). Arsenate adsorption on waste eggshell modified by goethite, α-MnO2 and goethite/α-MnO2. Chemical Engineering Journal, 237, 430–442.
Matschullat, J. (2000). Arsenic in the geosphere—a review. Science of the Total Environment, 249, 297–312.
Mondal, P., Majumder, C. B., & Mohanty, B. (2007). A laboratory study for the treatment of arsenic, iron, and manganese bearing ground water using Fe+3 impregnated activated carbon: effects of shaking time, pH and temperature. Journal of Hazardous Materials, 144(1–2), 420–426.
Murphy, E. M., Zachara, J. M., Smith, S. C., & Phillips, J. L. (1992). The sorption of humic acids to mineral surfaces and their role in contaminant binding. Science of the Total Environment, 117/118, 413–423.
Najm, I. N., Patania, N. L., Jacangelo, J. G., & Krasner, S. W. (1994). Evaluating surrogates for disinfection by-products (DBPs). Journal AWWA, 86(6), 98–106.
Nayak, B., Amir Hossain, M. D., Sengupta, M. K., Ahamed, S., Das, B., Pal, A., & Mukherjee, A. (2006). Adsorption studies with arsenic onto ferric hydroxide gel in a non-oxidizing environment: the effect of co-occurring solutes and speciation. Water Quality Research Journal Of Canada, 41(3), 333–340.
Nienow, A. W. (1969). Dissolution mass transfer in a turbine agitated baffled vessel. Canadian Journal of Chemical Engineering, 4, 248–258.
Pan, Y. F., Chiou, C. T., & Lin, T. F. (2010). Adsorption of As(V) by iron-oxide-coated diatomite (IOCD). Environmental Science and Pollution Research, 17, 1401–1410.
Peng, X., Luan, Z., & Zhang, H. (2006). Montmorillonite Cu(II)/Fe(III) oxides magnetic material as adsorbent for removal of humic acid and its thermal regeneration. Chemosphere, 63, 300–306.
Pierce, M. L., & Moore, C. B. (1982). Adsorption of arsenite and arsenate on amorphous iron hydroxide. Water Research, 16, 1247–1253.
Ramesh, A., Hasegawa, H., Maki, T., & Ueda, K. (2007). Adsorption of inorganic and organic arsenic from aqueous solutions by polymeric Al/Fe modified montmorillonite. Separation and Purification Technology, 56(1), 90–100.
Raven, K. P., Jain, A., & Loeppert, R. H. (1998). Arsenite and arsenate adsorption on ferrihydrite: kinetics, equilibrium, and adsorption envelopes. Environmental Science and Technology, 32, 344–349.
Redman, A. D., Macalady, D. L., & Ahmann, D. (2002). Natural organic matter affects As speciation and sorption onto hematite. Environmental Science and Technology, 36, 2889–2896.
Rodrigues, A., Brito, A., Janknecht, P., Proenc, M. F., & Nogueira, R. (2008). Quantification of humic acids in surface water: effects of divalent cations, pH, and filtration. Journal of Environmental Monitoring, 11, 377–382.
Shinozuka, T., Shibata, M., & Yamaguchi, T. (2004). Molecular weight characterization of humic substances by MALDI-TOF-MS. Journal of Mass Spectrometry Society of Japan, 52(1), 29–32.
Simsek, E. S., Özdemir, E., & Beker, U. (2013). Process optimization for As adsorption onto natural zeolite incorporating metal oxides by response surface methodology. Water, Air, and Soil Pollution, 224, 1614.
Singh, D. B., Prasad, G., Rupainwar, D. C., & Singh, V. N. (1988). As(III) removal from aqueous solution by adsorption. Water, Air, and Soil Pollution, 42, 373–386.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Summers, R. S., Haist, B., Koehler, J., Ritz, J., Zimmers, G., & Sontheimer, H. (1989). The influence of background organic matter on GAC adsorption. Journal of American Water Works Association, 81(5), 66–72.
Sun, X., & Doner, H. E. (1998). Adsorption and oxidation of arsenite on goethite. Soil Science, 163, 278–287.
Tamaki, S., & Frankenberger, W. T. (1992). Environmental biochemistry of arsenic. Reviews of Environmental Contamination and Toxicology, 124, 79–110.
Tien. C. (1994). Adsorption calculation and modeling. Butterworth-Heinemann, MA.
TWEPA. (2014). National drinking water quality standards. Taipei, Taiwan: TWEPA.
Urbano, B. F., Rivas, B. L., Martinez, F., & Alexandratos, S. D. (2012). Equilibrium and kinetic study of As sorption by water-insoluble nanocomposite resin of poly[N-(4-vinylbenzyl)-N-methyl-D-glucamine]- montmorillonite. Chemical Engineering Journal, 93(194), 21–30.
USEPA, (1999). Technologies and costs for removal of As from drinking water. EPA 815: P-01-001.
USEPA. (2001). National primary drinking water regulations: As and clarifications to compliance and new source contaminants monitoring. Federal Register, 14, 6975–7066.
Wilkie, J. A., & Hering, J. G. (1996). Adsorption of arsenic onto ferric hydroxide: effects of adsorbate/adsorbent ratios and co-occurring solutes. Colloids Surface A, 107, 97–110.
Yoshida, T., Yamauchi, H., & Sun, G. F. (2004). Chronic health effects in people exposed to As via the drinking water: dose–response relationships in review. Toxicology and Applied Pharmacology, 198, 243–252.
Yu, X. (2001). Humic acids from endemic arsenicosis areas in inner Mongolia and from the blackfoot diseaseareas in Taiwan: a comparative study. Environmental Geochemistry and Health, 23, 27–42.
Yuan, J. R., Ghosh, M. M., & Teoh, R. S. (1987). Adsorption of As on hydrous oxides. In: Kolaczkowski S.T.,Crittenden B.D., editors. Management of Hazardous and Toxic Wastes in the Process Industries (pp. 363–371). London, UK: Elsevier Applied Science
Zeng, L. (2004). Arsenic adsorption from aqueous solutions on an Fe(III)-Si binary oxide adsorbent. Water Quality Research Journal, 39(3), 267–275.
Zhou, W., Fu, H., Pan, K., Tian, C., Qu, Y., Lu, P., & Sun, C. C. (2008). Mesoporous TiO2/α-Fe2O3: bifunctional composites for effective elimination of arsenite contamination through simultaneous photocatalytic oxidation and adsorption. The Journal of Physical Chemistry C, 112, 19584–19589.
Acknowledgments
This research is supported by Taiwan National Science Council under Grant Number NSC 100-2221-E-006-036-MY3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fakour, H., Pan, YF. & Lin, TF. Effect of Humic Acid on Arsenic Adsorption and Pore Blockage on Iron-Based Adsorbent. Water Air Soil Pollut 226, 14 (2015). https://doi.org/10.1007/s11270-014-2224-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2224-2