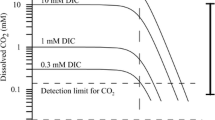
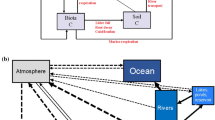
Abstract
Measurement of the concentration of dissolved carbon dioxide in ground and surface aqueous environments is needed for a wide variety of scientific and industrial applications. These environments can be fresh, saline, or transitional in nature and can be hydrochemically complex. A next generation of sensors, like fiber-optic sensors, offer real-time, direct, distributed sensing of dissolved carbon dioxide and are an improvement over current technology for many applications; however, these sensors may be susceptible to signal disturbance when deployed in hydrochemically complex, natural environments. This complexity can best be characterized using hydrochemical modeling techniques. The modeling software, phreeqc 2.18, was used to conduct a comprehensive review to gain perspective on published data of natural water samples. Freshwater, saltwater, and transitional environments were characterized in terms of the distribution of carbonate and non-carbonate species present. Saline, transitional, and deep freshwater environments had the broadest range of carbonate distribution and species that may cross-interfere with sensor response. These data should be used to build complex laboratory test solutions that mimic the natural environment for use in sensor development. In some cases, specially engineered membranes may be required to mitigate the potentially cross-interfering effect of these ions.






Similar content being viewed by others
References
Abu-Jaber, N.S., & Wafa, N.A. (1996). Hydrochemistry of aquifers in the southern Dead Sea area, southern Jordan. Hydrochemistry of aquifers in the southern Dead Sea area, southern Jordan. Environmental Geology, 28(4), 213–222.
Almeida, F., Guimaraes, J., Jardim, W. (2001). Measuring the CO2 flux at the air/water interface in lakes using flow injection analysis. Journal of Environmental Monitoring, 3(3), 317–321.
Appelo, C.A.J., & Postma, D. (2005). Geochemistry, groundwater and pollution, 2nd edn. Amsterdam.
Bäckström, M., Nilsson, U., Hȧkansson, K., Allard, B., Karlsson, S. (2003). Speciation of heavy metals in road runoff and roadside total deposition. Water, Air, and Soil Pollution, 147(1–4), 343–366.
Bao, B., Melo, L., Davies, B., Fadaei, H., Sinton, D., Wild, P. (2013). Detecting supercritical CO2 in brine at sequestration pressure with an optical fiber sensor. Environmental Science & Technology, 47(1), 306–313.
Benson, S.M., & Surles, T. (2006). Carbon capture and storage: an overview with emphasis on capture and storage in deep geological formations. In Proceedings of the IEEE.
Birkle, P., & Aragon, J. (2002). Evolution and origin of deep reservoir water at the Activo Luna oil field, Gulf of Mexico, Mexico. AAPG Bulletin, 3(3), 457–484.
Bradshaw, A.L., Brewer, P.G., Shafer, D.K., Williams, R.T. (1981). Measurements of total carbon dioxide and alkalinity by potentiometric titration in the GEOSECS program. Earth and Planetary Science Letters, 55(1), 99–115.
Bruland, K., & Lohan, M. (2006). Controls of trace metals in seawater In Elderfield, H., Holland, H., T. K. K. (Eds.), The Oceans and Marine Geochemistry, (pp. 23-47). Elsevier.
Carol, E.S., & Kruse, E.E. (2012). Hydrochemical characterization of the water resources in the coastal environments of the outer Río de la Plata estuary, Argentina. Journal of South American Earth Sciences, 37, 113–121.
Centeno, T.A., & Fuertes, A.B. (2001). Carbon molecular sieve membranes derived from a phenolic resin supported on porous ceramic tubes. Separation and Purification Technology, 25(1), 379–384.
Cowell, D., & Ford, D. (1980). Hydrochemistry of a dolomite karst: the Bruce Peninsula of Ontario. Canadian Journal of Earth Sciences.
Dickson, A.G., Sabine, C.L., Christian, J.R. (2007). Guide to best practices for ocean CO 2 measurements: PICES Special Publication.
Doney, S.C., Fabry, V.J., Feely, R. a., Kleypas, J. a (2009). Ocean acidification: the other CO 2 problem. Annual Review of Marine Science, 1(1), 169–192.
Dotsika, E., Poutoukis, D., Michelot, J., Raco, B. (2009). Natural tracers for identifying the origin of the thermal fluids emerging along the Aegean Volcanic arc (Greece): Evidence of Arc-Type Magmatic Water (ATMW) participation. Journal of Volcanology and Geothermal Research, 179(1–2), 19–32.
Friedrichs, G., Bock, J., Temps, F., Fietzek, P., Körtzinger, A., Wallace, D.W. (2010). Toward continuous monitoring of seawater 13CO2/12CO2 isotope ratio and pCO 2: performance of cavity ringdown spectroscopy and gas matrix effects. Limnology Oceanography.: Methods, 8, 539–551.
Goyet, C., Walt, D.R., Brewer, P.G. (1992). Development of a fiber-optic sensor for measurement of pCO2 in sea water: design criteria and sea trials. Deep Sea Research Part A. Oceanographic Research Papers, 39(6), 1015–1026.
Hidalgo, M, & Cruz-Sanjulián, J. (2001). Groundwater composition, hydrochemical evolution and mass transfer in a regional detrital aquifer (Baza basin, southern Spain). Applied Geochemistry, 16, 745–758.
Kim, Y., Lee, K.-S., Koh, D.-C., Lee, D.-H., Lee, S.-G., Park, W.-B., et al. (2003). Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: a case study in Jeju volcanic island, Korea. Journal of Hydrology, 270(34), 282–294.
Liu, X., Byrne, R.H., Adornato, L., Yates, K.K., Kaltenbacher, E., Ding, X., et al. (2013). In situ spectrophotometric measurement of dissolved inorganic carbon in seawater. Environmental Science & Technology, 47(19), 11106–11114.
Lucklum, R., Henning, B., Hauptmann, P., Schierbaum, K.D., Vaihinger, S., Gopel, W. (1991). Quartz microbalance sensors for gas detection. Sensors and Actuators, 27, 705–710.
Lueker, T.J., Dickson, A.G., Keeling, C.D. (2000). Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: validation based on laboratory measurements of CO 2 in gas and seawater at equilibrium. Marine Chemistry, 70(1–3), 105–119.
Mosello, R. (1984). Hydrochemistry of high altitude alpine lakes. Schweizerische Zeitschrift für Hydrologie, 46, 86–99.
Orghici, R., Willer, U., Gierszewska, M., Waldvogel, S., Schade, W. (2008). Fiber-optic evanescent field sensor for detection of explosives and CO 2 dissolved in water. Applied Physics B, 90(2), 355–360.
Papatheodorou, G., Demopoulou, G., Lambrakis, N. (2006). A long-term study of temporal hydrochemical data in a shallow lake using multivariate statistical techniques. Ecological Modelling, 193(34), 759–776.
Parkhurst, D.L., & Appelo, C. (1999). Users guide to PHREEQC (version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations.
Puckett, L., Cowdery, T., McMahon, P., Tornes, L., Stoner, J. (2002). Using chemical, hydrologic, and age dating analysis to delineate redox processes and flow paths in the riparian zone of a glacial outwash aquifer-stream system. Water Resources Research, 38(8), 9–29.
Reimer, A., Landmann, G., Kempe, S. (2008). Lake Van, Eastern Anatolia, Hydrochemistry and History. Aquatic Geochemistry, 15(1–2), 195–222.
Schuster, U., Hannides, a., Mintrop, L., Körtzinger, A (2009). Sensors and instruments for oceanic dissolved carbon measurements. Ocean Science Discussions, 6(1), 491–524.
Siegert, M.J., Tranter, M., Ellis-Evans, J.C., Priscu, J.C., Berry Lyons, W. (2003). The hydrochemistry of lake vostok and the potential for life in antarctic subglacial lakes. Hydrological processes, 17(4), 795–814.
Smart, R., Soulsby, C., Neal, C., Wade, A., Cresser, M., Billett, M., et al. (1998). Factors regulating the spatial and temporal distribution of solute concentrations in a major river system in NE Scotland. The Science of The Total Environment, 221(2–3), 93–110.
Striegl, R.G., Kortelainen, P., Chanton, J.P., Wickland, K.P., Bugna, G.C., Rantakari, M. (2001). Carbon dioxide partial pressure and 13C content of North Temperate and Boreal Lakes at Spring Ice Melt. Limnology and Oceanography, 46(4), 941–945.
Stumm, W., & Morgan, J. (1996). Aquatic chemistry; chemical equilibria and rates in natural waters, 3rd. New York: Wiley Interscience.
Takahashi, T., Weiss, R.F., Culberson, C., Edmond, J., Hammond, D., Wong, C., et al. (1970). The alkalinity and total carbon dioxide concentration in the world oceans. Journal of Geophysical Research, 75, 7648–7666.
Takahashi, T., Broecker, W., Bainbridge, A. (1981). The alkalinity and total carbon dioxide concentration in the world oceans. Carbon Cycle Modelling, SCOPE, 16(3078), 271–286.
Themann, S., Schmidt, H.M., Esser, D. (2009). SPE-129127-MS measurement, monitoring, and verification of CO 2 storage: An integrated approach. In Proceedings of SPE International Conference on CO2 Capture, Storage, and Utilization. San Diego: Society of Petroleum Engineers.
Wium-Andersen, S., & Andersen, J.M. (1971). Carbon dioxide content of the interstitial water in the sediment of Grane Langso, a Danish Lobelia lake. American Society of Limnology and Oceanography, 17(6), 943–947.
Wolfbeis, O.S. (2000). Fiber-optic chemical sensors and biosensors. Analytical Chemistry, 72, 81–89.
Wolf-Gladrow, D., Zeebe, R., Klaas, C., Körtzinger, A., Dickson, A.G. (2007). Total alkalinity: the explicit conservative expression and its application to biogeochemical processes. Marine Chemistry, 106(1–2), 287–300.
Acknowledgments
The authors would like to thank the Carbon Management Canada, the National Science and Engineering Council, Drs. Peter Wild, David Sinton, Don Lawton, Martin Jun, Ernie Perkins, Bernhard Mayer, Shannon Sterling and Geoff Burton, Luis Melo, Ben Davies, and Bo Bao.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhatia, S., Risk, D. Speciation in Application Environments for Dissolved Carbon Dioxide Sensors. Water Air Soil Pollut 226, 154 (2015). https://doi.org/10.1007/s11270-014-2200-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2200-x