Abstract
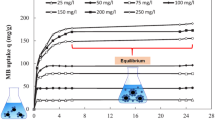
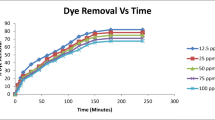
A common weed, Imperata cylindrica (cogongrass), was used as a low-cost adsorbent for the adsorption of methylene blue (MB) and the process optimized. The effects of four factors, namely, shaking speed (100–300 rpm), pH (3–9), contact time (10–40 min) and adsorbent dosage (0.4–1.0 g), on colour removal and chemical oxygen demand (COD) reduction of MB were studied and optimized using fractional factorial design and response surface methodology. The two factors that play a vital role in the adsorption process are pH and adsorbent dosage. From the results, colour removal and COD reduction recorded coefficient of determination (r 2) values of 0.9600 and 0.9594, respectively. Optimum adsorption conditions, resulting in 99.09 % colour removal and 97.87 % COD reduction, were achieved at shaking speed of 100 rpm, pH 9, 40 min contact time and adsorbent dosage of 1.0 g. The adsorption systems for MB dye were found to fit the pseudo-second order model instead of the pseudo-first order model, while equilibrium studies showed that the adsorption process followed the Langmuir isotherm.






Similar content being viewed by others
References
Alaya, M. N., Hourieh, M. A., Youssef, A. M., & El-Sejariah, F. (2000). Adsorption properties of activated carbons prepared from olive stones by chemical and physical activation. Adsorption Science & Technology, 18(1), 27–42. doi:10.1260/0263617001493251.
Annadurai, G., Juang, R. S., & Lee, D. J. (2002). Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials, 92(3), 263–274. doi:10.1016/S0304-3894(02)00017-1.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington DC: American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF).
Chowdhury, S., Mishra, R., Saha, P., & Kushwaha, P. (2011). Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination, 265(1–3), 159–168. doi:10.1016/j.desal.2010.07.047.
Dogan, M., Abak, H., & Alkan, M. (2009). Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. Journal of Hazardous Materials, 164(1), 172–181. doi:10.1016/j.jhazmat.2008.07.155.
Dutta, S., Bhattacharyya, A., Ganguly, A., Gupta, S., & Basu, S. (2011). Application of response surface methodology for preparation of low-cost adsorbent from citrus fruit peel and for removal of methylene blue. Desalination, 275(1–3), 26–36. doi:10.1016/j.desal.2011.02.057.
Ghosh, D., & Bhattacharyya, K. G. (2002). Adsorption of methylene blue on kaolinite. Applied Clay Science, 20(6), 295–300. doi:10.1016/S0169-1317(01)00081-3.
Gong, R. M., Li, M., Yang, C., Sun, Y. Z., & Chen, J. (2005). Removal of cationic dyes from aqueous solution by adsorption on peanut hull. Journal of Hazardous Materials, 121(1–3), 247–250. doi:10.1016/j.jhazmat.2005.01.029.
Gupta, V. K., & Suhas. (2009). Application of low-cost adsorbents for dye removal—a review. Journal of Environmental Management, 90(8), 2313–2342. doi:10.1016/j.jenvman.2008.11.017.
Hameed, B. H. (2009a). Grass waste: a novel sorbent for the removal of basic dye from aqueous solution. Journal of Hazardous Materials, 166(1), 233–238. doi:10.1016/j.jhazmat.2008.11.019.
Hameed, B. H. (2009b). Spent tea leaves: a new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. Journal of Hazardous Materials, 161(2–3), 753–759. doi:10.1016/j.jhazmat.2008.04.019.
Hameed, B. H., Din, A. T. M., & Ahmad, A. L. (2007). Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. Journal of Hazardous Materials, 141(3), 819–825. doi:10.1016/j.jhazmat.2006.07.049.
Hameed, B. H., Mahmoud, D. K., & Ahmad, A. L. (2008). Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: coconut (Cocos nucifera) bunch waste. Journal of Hazardous Materials, 158(1), 65–72. doi:10.1016/j.jhazmat.2008.01.034.
Hanafiah, M. A. K. M., Zakaria, H., & Ngah, W. S. W. (2010). Base treated cogon grass (Imperata cylindrica) as an adsorbent for the removal of Ni(II): kinetic, isothermal and fixed-bed column studies. Clean-Soil Air Water, 38(3), 248–256. doi:10.1002/clen.200900206.
Ho, Y. S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70(2), 115–124. doi:10.1016/S0923-0467(98)00076-1.
Janos, P., Coskun, S., Pilarova, V., & Rejnek, J. (2009). Removal of basic (Methylene Blue) and acid (Egacid Orange) dyes from waters by sorption on chemically treated wood shavings. Bioresource Technology, 100(3), 1450–1453. doi:10.1016/j.biortech.2008.06.069.
Joshi, K. M., & Shrivastava, J. S. (2011). Removal of methylene blue dye aqueous solution using photocatalysis. International Journal of Nano Dimension, 2(4), 241–252.
Khattri, S. D., & Singh, M. K. (2009). Removal of malachite green from dye wastewater using neem sawdust by adsorption. Journal of Hazardous Materials, 167(1–3), 1089–1094. doi:10.1016/j.jhazmat.2009.01.101.
Khelifi, E., Gannoun, H., Touhami, Y., Bouallagui, H., & Hamdi, M. (2008). Aerobic decolourization of the indigo dye-containing textile wastewater using continuous combined bioreactors. Journal of Hazardous Materials, 152(2), 683–689. doi:10.1016/j.jhazmat.2007.07.059.
Lagergren, S. Y. (1898). Zur theorie der sogenannten adsoprtion gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar, 24(4), 1–39.
Li, Z. M., Teng, T. T., Alkarkhi, A. F. M., Rafatullah, M., & Low, L. W. (2013). Chemical modification of Imperata cylindrica leaf powder for heavy metal ion adsorption. Water, Air, and Soil Pollution, 224(4), 1505. doi:10.1007/S11270-013-1505-5.
Liu, Y., Wang, J. T., Zheng, Y., & Wang, A. Q. (2012). Adsorption of methylene blue by kapok fiber treated by sodium chlorite optimized with response surface methodology. Chemical Engineering Journal, 184, 248–255. doi:10.1016/j.cej.2012.01.049.
Low, L. W., Teng, T. T., Ahmad, A., Morad, N., & Wong, Y. S. (2011a). A novel pretreatment method of lignocellulosic material as adsorbent and kinetic study of dye waste adsorption. Water, Air, and Soil Pollution, 218(1–4), 293–306. doi:10.1007/s11270-010-0642-3.
Low, L. W., Teng, T. T., Alkarkhi, A. F. M., Ahmad, A., & Morad, N. (2011b). Optimization of the adsorption conditions for the decolorization and COD reduction of methylene blue aqueous solution using low-cost adsorbent. Water, Air, and Soil Pollution, 214(1–4), 185–195. doi:10.1007/s11270-010-0414-0.
Low, L. W., Teng, T. T., Alkarkhi, A. F. M., Morad, N., & Azahan, B. (2013a). Adsorption of Rhodamine B dye on Elaeis guineensis frond fiber. Separation Science and Technology. doi:10.1080/01496395.2013.872148.
Low, L. W., Teng, T. T., Morad, N., & Azahari, B. (2013b). Optimization of the column studies into the adsorption of basic dye using tartaric acid-treated bagasse. Desalination and Water Treatment. doi:10.1080/19443994.2013.817630.
Low, L. W., Teng, T. T., Rafatullah, M., Morad, N., & Azahari, B. (2013c). Adsorption studies of methylene blue and malachite green from aqueous solutions by pretreated lignocellulosic materials. Separation Science and Technology, 48(11), 1688–1698. doi:10.1080/01496395.2012.756912.
Mane, V. S., & Babu, P. V. V. (2011). Studies on the adsorption of Brilliant Green dye from aqueous solution onto low-cost NaOH treated saw dust. Desalination, 273(2–3), 321–329. doi:10.1016/j.desal.2011.01.049.
Mendez-Paz, D., Omil, F., & Lema, J. M. (2005). Anaerobic treatment of azo dye Acid Orange 7 under fed-batch and continuous conditions. Water Research, 39(5), 771–778. doi:10.1016/j.watres.2004.11.022.
Mohan, D., Singh, K. P., Singh, G., & Kumar, K. (2002). Removal of dyes from wastewater using fly ash, a low-cost adsorbent. Industrial & Engineering Chemistry Research, 41(15), 3688–3695. doi:10.1021/Ie010667+.
Muthuraman, G., & Teng, T. T. (2009). Extraction and recovery of rhodamine B, methyl violet and methylene blue from industrial wastewater using D2EHPA as an extractant. Journal of Industrial and Engineering Chemistry, 15(6), 841–846. doi:10.1016/j.jiec.2009.09.010.
Muthuraman, G., Teng, T. T., Leh, C. P., & Norli, I. (2009). Use of bulk liquid membrane for the removal of chromium (VI) from aqueous acidic solution with tri-n-butyl phosphate as a carrier. Desalination, 249(2), 884–890. doi:10.1016/j.desal.2009.09.008.
Noordin, M. Y., Venkatesh, V. C., Sharif, S., Elting, S., & Abdullah, A. (2004). Application of response surface methodology in describing the performance of coated carbide tools when turning AISI 1045 steel. Journal of Materials Processing Technology, 145(1), 46–58. doi:10.1016/S0924-0136(03)00861-6.
Ozdemir, F. A., Demirata, B., & Apak, R. (2009). Adsorptive removal of methylene blue from simulated dyeing wastewater with melamine-formaldehyde-urea resin. Journal of Applied Polymer Science, 112(6), 3442–3448. doi:10.1002/App.29835.
Panizza, M., & Cerisola, G. (2008). Removal of colour and COD from wastewater containing acid blue 22 by electrochemical oxidation. Journal of Hazardous Materials, 153(1–2), 83–88. doi:10.1016/j.jhazmat.2007.08.023.
Sharma, Y. C., Uma, & Upadhyay, S. N. (2009). Removal of a cationic dye from wastewaters by adsorption on activated carbon developed from coconut coir. Energy & Fuels, 23, 2983–2988. doi:10.1021/Ef9001132.
Tan, B. H., Teng, T. T., & Omar, A. K. M. (2000). Removal of dyes and industrial dye wastes by magnesium chloride. Water Research, 34(2), 597–601. doi:10.1016/S0043-1354(99)00151-7.
Teng, T. T., & Low, L. W. (2012). Removal of dyes and pigments from industrial effluents. In S. K. Sharma & R. Sanghi (Eds.), Water treatment and pollution prevention: advances in research (pp. 64–93). New York: Springer.
Vargas, A. M. M., Cazetta, A. L., Kunita, M. H., Silva, T. L., & Almeida, V. C. (2011). Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chemical Engineering Journal, 168(2), 722–730. doi:10.1016/j.cej.2011.01.067.
Wang, X. S., Zhou, Y., Jiang, Y., & Sun, C. (2008). The removal of basic dyes from aqueous solutions using agricultural by-products. Journal of Hazardous Materials, 157(2–3), 374–385. doi:10.1016/j.jhazmat.2008.01.004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, C.XH., Teng, T.T., Alkarkhi, A.F.M. et al. Imperata cylindrica (Cogongrass) as an Adsorbent for Methylene Blue Dye Removal: Process Optimization. Water Air Soil Pollut 225, 1941 (2014). https://doi.org/10.1007/s11270-014-1941-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1941-x