Abstract
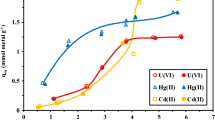
A novel sorbent, chitosan-immobilized pumice, has been prepared for the sorption of As(V) from waters prior to its determination by hydride generation atomic absorption spectrometry. The success of the immobilization has been checked with such characterization techniques as scanning electron microscopy, thermal gravimetric analysis, and elemental analysis. Points of zero charge of the sorbents were determined with potentiometric mass titration. Batch-type equilibration studies have shown that the novel sorbent can be employed at a wide pH range resulting in quantitative sorption (>90 %) at pH 3.0–7.0 and greater than 70 % sorption at pH >8.0. These results demonstrate the advantage of immobilizing chitosan onto pumice, because, under the same conditions, pumice displays <20 % sorption toward As(V), whereas chitosan gives approximately 90 % sorption only at pH 3.0. The validity of the method was verified through the analysis of ultrapure, bottled drinking, and tap water samples spiked with arsenate; the respective sorption percentages of 93.2 (±0.7), 89.0 (±1.0), and 80.9 (±1.3) were obtained by batch-type equilibration. Arsenic sorption was also examined in the presence of common interfering ions resulting in competing effects of PO4 3− and NO3 − on As(V) adsorption.






Similar content being viewed by others
References
Alemayehu, E., & Lennartz, B. (2009). Virgin volcanic rocks: kinetics and equilibrium studies for the adsorption of cadmium from water. Journal of Hazardous Materials, 169, 395–401.
Asgari, G., Roshani, B., & Ghanizadeh, G. (2012). The investigation of kinetic and isotherm of fluoride adsorption onto functionalize pumice stone. Journal of Hazardous Materials, 217–218, 123–132.
Atkins, P., & de Paula, J. (2002). Atkins’ physical chemistry. New York: Oxford University Press.
Baytak, S., Kenduzler, E., Turker, A. R., & Gok, N. (2008). Penicillium digitatum immobilized on pumice stone as a new solid phase extractor for preconcentration and/or separation of trace metals in environmental samples. Journal of Hazardous Materials, 153, 975–983.
Boddu, V. M., Abburi, K., Talbott, J. L., Smith, E. D., & Haasch, R. (2008). Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Research, 42, 633–642.
Bourikas, K., Vakros, J., & Kordulis, C. (2003). Potentiometric mass titrations: experimental and theoretical establishment of a new technique for determining the point of zero charge (PZC) of metal (hydr)oxides. Journal of Physical Chemistry B, 107, 9441–9451.
Boyacı, E., Eroğlu, A. E., & Shahwan, T. (2010). Sorption of As (V) from waters using chitosan and chitosan-immobilized sodium silicate prior to atomic spectrometric determination. Talanta, 80, 1452–1460.
Chatterjee, S., & Woo, S. H. (2009). The removal of nitrate from aqueous solutions by chitosan hydrogel beads. Journal of Hazardous Materials, 164, 1012–1018.
Chaunhan, D., Jaiswal, M., & Sankararamakrishnan, N. (2012). Removal of cadmium and hexavalent chromium from electroplating waste water using thiocarbamoyl chitosan. Carbohydrate Polymers, 88, 670–675.
Chen, C. C., & Chung, Y. C. (2006). Arsenic removal using a biopolymer chitosan sorbent. Journal of Environmental Science and Health Part A, 41, 645–658.
Chen, C. Y., Chang, T. H., Kuo, J. T., Chen, Y. F., & Chung, Y. C. (2008). Characteristics of molybdate-impregnated chitosan beads (MICB) in terms of arsenic removal from water and the application of a MICB-packed column to remove arsenic from wastewater. Bioresource Technology, 99, 7487–7494.
Choong, T. S. Y., Chuah, T. G., Robiah, Y., Koay, F. L. G., & Azni, I. (2007). Arsenic toxicity, health hazards and removal techniques from water: an overview. Desalination, 217, 139–166.
Dambies, L., Vincent, T., & Guibal, E. (2002). Treatment of arsenic-containing solutions using chitosan derivatives: uptake mechanism and sorption performances. Water Research, 36, 3699–3710.
Elwakeel, K. Z. (2010). Environmental application of chitosan resins for the treatment of water and wastewater: a review. Journal of Dispersion Science and Technology, 31, 273–288.
Ersoy, B., Sariisik, A., Dikmen, S., & Sariisik, G. (2010). Characterization of acidic pumice and determination of its electrokinetic properties in water. Powder Technology, 197, 129–135.
Guibal, E., Milot, C., & Tobin, J. M. (1998). Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Industrial and Engineering Chemistry Research, 37, 1454–1463.
Hansen, H. K., Nunez, P., & Jil, C. (2008). Removal of arsenic from wastewaters by airlift electrocoagulation. Part 2: continuous reactor experiments. Separation Science and Technology, 43, 3663–3675.
Hasan, S., Ghosh, T. K., Viswanath, D. S., & Boddu, V. M. (2008). Dispersion of chitosan on perlite for enhancement of copper (II) adsorption capacity. Journal of Hazardous Materials, 152, 826–837.
Huang, C. P., & Fu, P. L. K. (1984). Treatment of Arsenic(V)-containing water by the activated carbon process. Journal WPCF, 56, 233–242.
Kavcar, P., Sofuoglu, A., & Sofuoglu, S. C. (2009). A health risk assessment for exposure to trace metals via drinking water ingestion pathway. International Journal of Hygiene and Environmental Health, 212, 216–227.
Kitis, M., & Kaplan, S. S. (2007). Advanced oxidation of natural organic matter using hydrogen peroxide and iron-coated pumice particles. Chemosphere, 68, 1846–1853.
Kitis, M., Karakaya, E., & Yigit, N. O. (2005). Heterogeneous catalytic degradation of cyanide using copper-impregnated pumice and hydrogen peroxide. Water Research, 39, 1652–1662.
Kundu, S., Kavalakatt, S. S., & Pal, A. (2004). Removal of arsenic using hardened paste of Portland cement: batch adsorption and column study. Water Research, 38, 3780–3790.
Luu, T. T. G., Sthiannopkao, S., & Kim, K. W. (2009). Arsenic and other trace elements contamination in groundwater and a risk assessment study for the residents in the Kandal Province of Cambodia. Environmental International, 35, 455–460.
Maliyekkal, S. M., Philip, L., & Pradeep, T. (2009). As (III), removal from drinking water using manganese oxide-coated-alumina: performance evaluation and mechanistic details of surface binding. Chemical Engineering Journal, 153, 101–107.
Miretzky, P., & Cirelli, A. F. (2009). Hg (II) removal from water by chitosan and chitosan derivatives: a review. Journal of Hazardous Materials, 167, 10–23.
Mohan, D., & Pittman, C. U., Jr. (2007). Arsenic removal from water/wastewater using adsorbents—a critical review. Journal of Hazardous Materials, 142, 1–53.
Panuccio, M. R., Sorgona, A., Rizzo, M., & Cacco, G. (2009). Cadmium adsorption on vermiculite, zeolite and pumice: batch experimental studies. Journal of Environmental Management, 90, 364–374.
Quintelas, C., Rocha, Z., Silva, B., Fonseca, B., Figueiredo, H., & Tavares, T. (2009). Removal of Cd (II), Cr (VI), Fe (III) and Ni (II) from aqueous solutions by an E. coli biofilm supported on kaolin. Chemical Engineering Journal, 149, 319–324.
Rau, I., Gonzalo, A., & Valiente, M. (2003). Arsenic (V) adsorption by immobilized iron mediation. Modeling of the adsorption process and influence of the interfering ions. Reactive and Functional Polymers, 54, 85–94.
Schijf, J., & Ebling, A. M. (2010). Investigation of the ionic strength dependence of Ulva lactuca acid functional group pKas by manual alkalimetric titrations. Environmental Science and Pollution Research, 44, 1644–1649.
Şeker, A., Shahwan, T., Eroğlu, A. E., Yılmaz, S., Demirel, Z., & Dalay, M. C. (2008). Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead (II), cadmium (II) and nickel (II) ions on Spirulina platensis. Journal of Hazardous Materials, 154, 973–980.
Tolaimate, A., Desbrieres, J., Rhazi, M., Alagui, A., Vicendon, M., & Vottero, P. (2000). On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer, 41, 2463–2469.
Viswanathan, N., Sundaram, C. S., & Meenakshi, S. (2009). Removal of fluoride from aqueous solutions using protonated chitosan beads. Journal of Hazardous Materials, 161, 423–430.
Yavuz, M., Gode, F., Pehlivan, E., Ozmert, S., & Sharma, Y. C. (2008). An economic removal of Cu2+ and Cr3+ on the new adsorbents: pumice and polyacrylonitrile/pumice composite. Chemical Engineering Journal, 137, 435–461.
Zhang, S., Guo, Z., Xu, J., & Niu, H. (2011). Effect of environmental conditions on the sorption of radiocobalt from aqueous solution to treated eggshell as biosorbent. Journal of Radioanalytical and Nuclear Chemistry, 288, 121–130.
Acknowledgments
The authors thank the Center of Material Research for the facilities SEM and TGA, Environmental Research Center for ICP-MS analyses in preliminary studies at İzmir Institute of Technology. Prof. Dr. Hürriyet Polat for particle size determination, Dr. Hüseyin Özgener for elemental analysis, and Prof. Dr. Mehmet Kitis at Suleyman Demirel University (Turkey) for kindly providing the pumice samples are also acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Turan, D., Kocahakimoğlu, C., Boyacı, E. et al. Chitosan-Immobilized Pumice for the Removal of As(V) from Waters. Water Air Soil Pollut 225, 1931 (2014). https://doi.org/10.1007/s11270-014-1931-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1931-z