Abstract
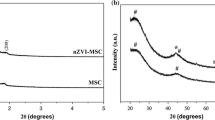
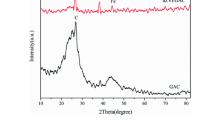
Nanoscale zero-valent iron supported on activated carbon (NZVI/AC) was synthesized by a modified potassium borohydride reduction method and characterized with X-ray diffraction (XRD), transmission electron microscopy (TEM), and specific surface area (SSA). The effects of NZVI loading on AC, NZVI/AC dosage, pH, the initial concentration of Cr(VI), and temperature on the removal of Cr(VI) were investigated. XRD confirmed the existence of Fe0 and TEM revealed that the material consisted of mainly spherical bead-like particles aggregated into chains of individual units. The SSA of the iron particles and the removal efficiency of Cr(VI) indicated that the optimum iron loading was 25 %. Increase of NZVI/AC dosage and reaction concentration abated the removal of Cr(VI). Kinetics studies showed that removal of Cr(VI) is a two-step reaction and each step could be expressed by pseudo-first-order reaction kinetics, with initial Cr(VI) and temperature as variables. Total Cr was always almost equal to that of Cr(VI) under all tested conditions, which indicated that little Cr(III) existed in solution. Iron ions, which could cause secondary pollution in the environment, are almost not released from this system. These results demonstrated that NZVI/AC could potentially be used for Cr(VI) removal.









Similar content being viewed by others
References
Alidokht, L., Khataee, A. R., & Reyhanitabar, A. (2011). Reductive removal of Cr(VI) by starch-stabilized Fe-0 nanoparticles in aqueous solution. Desalination, 270(1–3), 105–110.
Chen, S. S., Cheng, C. Y., Li, C. W., Chai, P. H., & Chang, Y. M. (2006). Reduction of chromate from electroplating wastewater from pH 1 to 2 using fluidized zero valent iron process. Journal of Hazardous materials, 142(1–2), 362–367.
Crane, R. A., & Scott, T. B. (2011). Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. Journal of Hazardous materials, 211–212(15), 112–125.
Eary, L. E., & Rai, D. (1988). Chromate removal from aqueous wastes by reduction with ferrous ion. Environmental Science and Technology, 22(8), 972–977.
Fang, Z. Q., Qiu, X. Q., & Huang, R. X. (2011). Removal of chromium in electroplating wastewater by nanoscale zero-valent metal with synergistic effect of reduction and immobilization. Desalination, 280(1–3), 224–231.
Geng, B., Jin, Z. H., Li, T. L., & Qi, X. H. (2009). Preparation of chitosan-stabilized Fe-0 nanoparticles for removal of hexavalent chromium in water. Science of The Total Environment, 407(18), 4994–5000.
Gheju, M. (2011). Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water, Air, and Soil Pollution, 222(1–4), 103–148.
He, Y., Gao, J. F., & Feng, F. Q. ( 2012). The comparative study on the rapid decolorization of azo, anthraquinone and triphenylmethane dyes by zero-valent iron. Chemical Engineering Journal, 179, 8–18.
Hoch, L. B., Mack, E. J., Hydutsky, B. W., Hershman, J. M., Skluzacek, J. M., & Mallouk, T. E. (2008). Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium. Environmental Science and Technology, 42(7), 2600–2605.
Li, Y. J., Gao, B. Y., Wu, T., Sun, D. J., Li, X., Wang, B., & Lu, F. J. (2009). Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide. Water Research, 43(12), 3067–3075.
Li, Y. M., Li, J. F., & Zhang, Y. L. (2012). Mechanism insights into enhanced Cr(VI) removal using nanoscale zerovalent iron supported on the pillared bentonite by macroscopic and spectroscopic studies. Journal of Hazardous materials, 227–228, 211–218.
Li, Z., Jones, H. K., Bowman, R. S., & Helferich, R. (1999). Enhanced reduction of chromate and PCE pelletized surfactant-modified zeolite/zerovalent iron. Environmental Science and Technology, 33(23), 4326–4330.
Liu, T. Y., Zhao, L., & Wang, Z. L. (2012). Removal of hexavalent chromium from wastewater by Fe-0-nanoparticles-chitosan composite beads: characterization, kinetics and thermodynamics. Water Science and Technology, 66(5), 1044–1051.
Liu, W. F., Zhang, J., & Zhang, C. L. (2010). Adsorptive removal of Cr (VI) by Fe-modified activated carbon prepared from Trapa natans husk. Chemical Engineering Journal, 162(2), 677–684.
Loacutepez-Teacutellez, G., Barrera-Diacuteaz, C. E., & Balderas-Hernaacutendez, P. (2011). Removal of hexavalent chromium in aquatic solutions by iron nanoparticles embedded in orange peel pith. Chemical Engineering Journal, 173(2), 480–485.
Long, T., & Ramsburg, C. A. (2011). Encapsulation of NZVI particles using a Gum Arabic stabilized oil-in-water emulsion. Journal of Hazardous materials, 189(3), 801–808.
Lowry, G. V., & Johnson, K. M. (2004). Congener-specific dechlorination of dissolved PCBs by microscale and nanoscale zerovalent iron in a water/methanol solution. Environmental Science and Technology, 38(19), 5208–5216.
Lv, X. S., Xu, J., & Jiang, G. M. (2011). Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere, 85(7), 1204–1209.
Mackenzie, K., Bleyl, S., Georgi, A., & Kopinke, F. D. (2012). Carbo-iron—an Fe/AC composite—as alternative to nano-iron for groundwater treatment. Water Research, 46(12), 3817–3826.
Mitra, P., Sarkar, D., & Chakrabarti, S. (2011). Reduction of hexa-valent chromium with zero-valent iron: batch kinetic studies and rate model. Chemical Engineering Journal, 171(1), 54–60.
Mohan, D., & Pittman, C. U. (2006). Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. Journal of Hazardous materials, 137(2), 762–811.
Noubactep, C. (2009). An analysis of the evolution of reactive species in Fe0/H2O systems. Journal of Hazardous materials, 168(2–3), 1626–1631.
Prasad, P. V. V. V., Das, C. D., & Golder, A. K. (2011). Reduction of Cr(VI) to Cr(III) and removal of total chromium from wastewater using scrap iron in the form of zerovalent iron (ZVI): batch and column studies. Canadian Journal of Chemical Engineering, 89(6), 1575–1582.
Schlautman, M. A., & Han, I. (2001). Effects of pH and dissolved oxygen on the reduction of hexavalent chromium by dissolved ferrous iron in poorly buffered aqueous systems. Water Research, 35(6), 1534–1546.
Shi, L. N., Zhang, X., & Chen, Z. L. (2011). Removal of chromium (VI) from waste water using bentonite-supported nanoscale zero-valent iron. Chemical Engineering Journal, 45(2), 886–892.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters. New York: Wiley.
Sun, Y., Takaoka, M., Takeda, N., Matsumoto, T., & Oshita, K. (2006). Kinetics on the decomposition of polychlorinated biphenyls with activated carbon-supported iron. Chemosphere, 65(2), 183–189.
Wu, Y. J., Zhang, J. H., & Tong, Y. F. (2009). Chromium (VI) reduction in aqueous solutions by Fe3 O4—stabilized Fe0 nanoparticles. Journal of Hazardous materials, 172(2–3), 1640–1645.
Xu, C. H., Cheng, D. D., Gao, B. Y., Yin, Z. l., Yue, Q. Y., & Zhao, X. (2012). Preparation and characterization of β-FeOOH-coated sand and its adsorption of Cr(VI) from aqueous solutions. Frontiers of Environmental Science and Engineering, 6(4), 455–462.
Yang, K., & Xing, B. S. (2009). Adsorption of fulvic acid by carbon nanotubes from water. Environmental Pollution, 157(4), 1095–1100.
Yoon, I. H., Bang, S., & Chang, J. S. (2010). Effects of pH and dissolved oxygen on Cr(VI) removal in Fe(0)/H2O systems. Journal of Hazardous materials, 186(1), 855–862.
Yuan, P., Fan, M. D., & Yang, D. (2009). Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. Journal of Hazardous materials, 166(2–3), 821–829.
Zhang, W. X. (2003). Nanoscale iron particles for environmental remediation: an overview. Journal of Nanoparticle Research, 5(3–4), 323–332.
Zhang, Y. L., Li, Y. M., & Li, J. F. (2012). Enhanced Cr(VI) removal by using the mixture of pillared bentonite and zero-valent iron. Chemical Engineering Journal, 185–186(15), 243–249.
Zou, X. H., Pan, J. M., Ou, H. X., Wang, X., Guan, W., Li, C. X., Yan, Y. S., & Duan, Y. Q. (2011). Adsorptive removal of Cr(III) and Fe(III) from aqueous solution by chitosan/attapulgite composites: equilibrium, thermodynamics and kinetics. Chemical Engineering Journal, 167(1), 112–121.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (grant no. 51102157) and Plans for Science and Technology Development of Shandong Province, China (grant no. 2013GSF11708). This study was also funded by National Major Special Technological Programs Concerning Water Pollution Control and Management in the Twelfth Five-year Plan Period (No. 2012 ZX07203-004). The authors thank Dr. Pamela Holt for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, CH., Zhu, Lj., Wang, XH. et al. Fast and Highly Efficient Removal of Chromate from Aqueous Solution Using Nanoscale Zero-Valent Iron/Activated Carbon (NZVI/AC). Water Air Soil Pollut 225, 1845 (2014). https://doi.org/10.1007/s11270-013-1845-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1845-1