Abstract
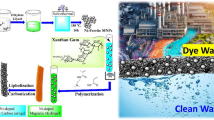
A novel mesoporous, nanocomposite, magnetically separable adsorbent, namely activated alumina (γ-Al2O3)/ferrite (Ni0.5Zn0.5Fe2O4) microfibers have been successfully prepared by the sol–gel process. These nanocomposite γ-Al2O3/Ni0.5Zn0.5Fe2O4 microfibers are formed after calcination of the precursor at 450 °C for 3 h, and characterized with high aspect ratios and uniform diameters of 1–10 μm. In the nanocomposite γ-Al2O3/Ni0.5Zn0.5Fe2O4 microfibers, the spherical γ-Al2O3 particles are homogeneously embedded on the microfiber. Their specific surface areas and magnetic properties are significantly influenced by the γ-Al2O3 content and calcination conditions. With the designed γ-Al2O3 mass fraction of 0.2 and the calcination temperature of 550 °C, the γ-Al2O3/Ni0.5Zn0.5Fe2O4 microfibers possess a high specific surface area of 118.3 m2/g and saturation magnetization (M s) of 20.4 Am2 kg−1, respectively. The adsorption behaviors of the nanocomposite γ-Al2O3/Ni0.5Zn0.5Fe2O4 microfibers were examined using the Congo red and methyl blue dyes as the adsorbate. The adsorption kinetics, effects of the adsorbent dosage and solution pH, adsorption isotherms, and regeneration of the microfiber adsorbents were investigated. The pseudo-second-order model can be used to describe the adsorption kinetics. The resultant isotherm data are well fitted by the Temkin model, implying that the dyes adsorption on the γ-Al2O3/Ni0.5Zn0.5Fe2O4 microfibers is a multilayer adsorption combined with some degrees of chemical interactions. Considering the simple synthesis process, high adsorption and unique magnetic property, these mesoporous, magnetic, nanocomposite γ-Al2O3/Ni0.5Zn0.5Fe2O4 microfibers can be used as a highly efficient, fast, and convenient adsorbent for dyes removal.
Highlights
-
The magnetic mesoporous Al2O3/Ni0.5Zn0.5Fe2O4 microfibers were synthesized.
-
Adsorption kinetics and adsorption isotherms were investigated.
-
The separation, regeneration, and adsorption efficiency were enhanced.

The novel mesoporous, magnetic, nanocomposite-activated alumina (γ-Al2O3)/ferrite (Ni0.5Zn0.5Fe2O4) microfiber adsorbents can be used as an efficient, fast, and convenient tool for dyes removal from wastewater.








Similar content being viewed by others
References
Anbia, M., & Salehi, S. (2012). Removal of acid dyes from aqueous media by adsorption onto amino-functionalized nanoporous silica SBA-3. Dyes and Pigments, 94, 1–9.
Bai, C. P., Xiong, X. F., Gong, W. Q., Feng, D. X., Xian, M., Ge, Z. X., & Xu, N. (2011). Removal of rhodamine B by ozone-based advanced oxidation process. Desalination, 278, 84–90.
Bouna, L., Rhouta, B., Amjoud, M., Maury, F., Lafont, M. C., Jada, A., Senocq, F., & Daoudi, L. (2011). Synthesis, characterization and photocatalytic activity of TiO2 supported natural palygorskite microfibers. Applied Clay Science, 52, 301–311.
Cojocaru, P., Magagnin, L., Gomez, E., & Vallés, E. (2011). Nanowires of NiCo/barium ferrite magnetic composite by electrodeposition. Materials Letters, 65, 2765–2769.
Delaney, P., McManamon, C., Hanrahan, J. P., Copley, M. P., Holmes, J. D., & Morris, M. A. (2011). Development of chemically engineered porous metal oxides for phosphate removal. Journal of Hazardous Materials, 185, 382–391.
Ghaedi, M., Tavallali, H., Sharifi, M., Kokhdan, S. N., & Asghari, A. (2012). Preparation of low cost activated carbon from Myrtus communis and pomegranate and their efficient application for removal of Congo red from aqueous solution. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 86, 107–114.
Gupta, V. K., Kumar, R., Nayak, A., Saleh, T. A., & Barakat, M. A. (2013). Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Advances in Colloid and Interface Science, 193, 24–34.
Hameed, B. H., & Ahmad, A. A. (2009). Batch adsorption of methyl blue from aqueous solution by garlic peel, an agricultural waste biomass. Journal of Hazardous Materials, 164, 870–875.
Hashemian, S., & Foroghimoqhadam, A. (2014). Effect of copper doping on CoTiO3 ilmenite type nanoparticles for removal of congo red from aqueous solution. Chemical Engineering Journal, 235, 299–306.
Ho, Y. S. (2006). Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Research, 40, 119–125.
Kohler, D., Schneider, M., Krüger, M., Lehr, C.-M., Möhwald, H., & Wang, D. Y. (2011). Template-assisted polyelectrolyte encapsulation of nanoparticles into dispersible, hierarchically nanostructured microfibers. Advanced Materials, 23, 1376–1379.
Li, H., Zhang, L., Dai, H. X., & He, H. (2009). Facile synthesis and unique physicochemical properties of three-dimensionally ordered macroporous magnesium oxide, gamma-alumina, and ceria-zirconia solid solutions with crystalline mesoporous walls. Inorganic Chemistry, 48, 4421–4434.
Liu, R. J., Lu, Y., Shen, X. Q., Liang, Q. R., & Wang, Q. J. (2012). Arsenic (V) adsorption from aqueous solution on magnetic Fe0.2(Co20Ni80)0.8 alloy porous microfibers. Water, Air, and Soil Pollution, 223, 5365–5373.
Mahapatra, A., Mishra, B. G., & Hota, G. (2013). Adsorptive removal of Congo red dye from wastewater by mixed iron oxide-alumina nanocomposites. Ceramic International, 39, 5443–5451.
Mezohegyi, G., Zee, F. P., Font, J., Fortuny, A., & Fabregat, A. (2012). Towards advanced aqueous dye removal processes: a short review on the versatile role of activated carbon. Journal of Environmental Management, 102, 148–164.
Ofomaja, A. (2011). Kinetics and pseudo-isotherm studies of 4-nitrophenol adsorption onto mansonia wood sawdust. Industrial Crops and Products, 33, 418–428.
Panda, G. C., Das, S. K., & Guha, A. K. (2009). Jute stick powder as a potential biomass for the removal of Congo red and rhodamine B from their aqueous solution. Journal of Hazardous Materials, 164, 374–379.
Patil, B. N., Naik, D. B., & Shrivastava, V. S. (2011). Photocatalytic degradation of hazardous Ponceau-S dye from industrial wastewater using nanosized niobium pentoxide with carbon. Desalination, 269, 276–283.
Patra, A. K., Dutta, A., & Bhaumik, A. (2012). Self-assembled mesoporous γ-Al2O3 spherical nanoparticles and their efficiency for the removal of arsenic from water. Journal of Hazardous Materials, 201–202, 170–177.
Pradhan, A. C., Varadwaj, G. B., & Parida, K. M. (2013). Facile fabrication of mesoporous iron modified Al2O3 nanoparticles pillared montmorillonite nanocomposite: a smart photo-Fenton catalyst for quick removal of organic dyes. Dalton Transactions, 42, 15139–15149.
Rafatullah, M., Sulaiman, O., Hashim, R., & Ahmad, A. (2010). Adsorption of methylene blue on low-cost adsorbents: a review. Journal of Hazardous Materials, 177, 70–80.
Rahimi, R., Kerdari, H., Rabbani, M., & Shafiee, M. (2011). Synthesis, characterization and adsorbing properties of hollow Zn-Fe2O4 nanospheres on removal of Congo red from aqueous solution. Desalination, 280, 412–418.
Salima, A., Benaouda, B., Noureddine, B., & Duclaux, L. (2013). Application of ulva lactura and systoceira algae-based activated carbons to hazardous cationic dyes removal from industrial effluents. Water Research, 47, 3375–3388.
Soltani, R. D., Khataee, A. R., Safari, M., & Joo, S. W. (2013). Preparation of bio-silica/chitosan nanocomposite for adsorption of a textile dye in aqueous solutions. International Biodeterioration & Biodegradation, 85, 383–391.
Song, F. Z., Shen, X. Q., Liu, M. Q., & Xiang, J. (2010). Magnetic hard/soft nanocomposite ferrite aligned hollow microfibers and remanence enhancement. Journal of Colloid and Interface Science, 354, 413–416.
Stefańska, K. S., Nowacka, M., Radzimska, A. K., & Jesionowski, T. (2012). Preparation of hybrid pigments via adsorption of selected food dyes onto inorganic oxides based on anatase titanium dioxide. Dyes and Pigments, 94, 338–348.
Sun, H. M., Cao, L. Y., & Lu, L. H. (2011). Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Research, 4, 550–562.
Tajizadegan, H., Jafari, M., Rashidzadeh, M., & Saffar-Teluri, A. (2013). A high activity adsorbent of ZnO–Al2O3 nanocomposite particles: synthesis, characterization and dye removal efficiency. Applied Surface Science, 276, 317–322.
Verma, A. K., Dash, R. R., & Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management, 93, 154–168.
Wang, L., & Wang, A. (2007). Adsorption characteristics of Congo red onto the chitosan/montmorillonite nanocomposite. Journal of Hazardous Materials, 147, 979–985.
Wang, L. X., Li, J. C., Wang, Y. Q., Zhao, L. J., & Jiang, Q. (2012). Adsorption capability for Congo red on nanocrystalline MFe2O4(M = Mn, Fe, Co, Ni) spinel ferrites. Chemical Engineering Journal, 181–182, 72–79.
Yang, X. C., Liu, R. J., Shen, X. Q., & Song, F. Z. (2012). Magnetic properties and BSA adsorption of nano-Fe-embedded BaFe12O19 porous microfibers via organic gel-thermal selective reduction process. Journal of Sol–Gel Science and Technology, 63, 8–15.
Yousef, R. I., EI-Eswed, B., & Al-Muhtaseb, A. H. (2011). Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: kinetics, mechanism, and thermodynamics studies. Chemical Engineering Journal, 171, 1143–1149.
Yu, S. C., Chen, Z. W., Cheng, Q. B., Lü, Z. H., Liu, M. H., & Gao, C. J. (2012). Application of thin-film composite hollow fiber membrane to submerged nanofiltration of anionic dye aqueous solutions. Separation and Purification Technology, 88, 121–129.
Zhao, M. F., & Liu, P. (2009). Adsorption of methyl blue from aqueous solutions by modified expanded graphite powder. Desalination, 249, 331–336.
Zhu, H. Y., Jiang, R., Xiao, L., & Li, W. (2010). A novel magnetically separable γ-Fe2O3/crosslinked chitosan adsorbent: preparation, characterization and adsorption application for removal of hazardous azo dye. Journal of Hazardous Materials, 179, 251–257.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant nos. 51274106 and 51202091), the Natural Science Foundation of Jiangsu Provincial Higher Education (grant no. 12KJA430001), the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20103227110006), the Science and Technology Support Program of Jiangsu Province (grant nos. BE2012143 and BE2013071), the Jiangsu Province's Postgraduate Cultivation and Innovation Project (grant nos. CXZZ11_0557 and CXZZ13_0662), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Wang, Z., Jing, M. et al. Efficient Removal of Dyes from Aqueous Solution by Mesoporous Nanocomposite Al2O3/Ni0.5Zn0.5Fe2O4 Microfibers. Water Air Soil Pollut 225, 1819 (2014). https://doi.org/10.1007/s11270-013-1819-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1819-3