Abstract
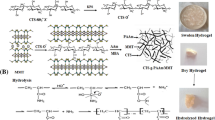
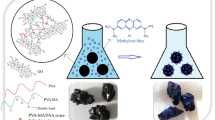
In this work, N-vinyl-2-pyrrolidone/itaconic acid/organo clay nanocomposite hydrogels were synthesized by free radical polymerization technique using different amounts of organo clay. Fourier transform infrared spectroscopy, X-ray diffraction, and scanning electron microscopy techniques were used for characterization of nanocomposite hydrogels and swelling, and mechanical properties of these hydrogels were investigated. Safranine-T adsorption capacities of nanocomposite hydrogels were investigated at different conditions such as pH, contact time, adsorbent dose and initial concentration of dye. The optimum pH value was found to be pH 6. According to the organo clay content, there are no significant differences in dye adsorption capacities of nanocomposite hydrogels until the clay content reaches 5 % wt. While the organo clay amount of nanocomposite hydrogels increases up to 10 % wt, dye adsorption capacities of these hydrogels significantly decrease. Adsorption processes of dye onto the nanocomposite hydrogels follow pseudo-second-order type adsorption kinetic. The equilibrium adsorption data have been evaluated using Freundlich and Langmuir Isotherm models. The results illustrated that the adsorption follows Langmuir isotherm.







Similar content being viewed by others
References
Acar, I., Bal, A., & Güçlü, G. (2012). Adsorption of basic dyes from aqueous solutions by depolymerization products of post-consumer PET bottles. Clean-Soil, Air, Water, 40, 325–333.
Akkaya, M. Ç., Emik, S., Güçlü, G., İyim, T. B., & Özgümüş, S. (2009). Removal of basic dyes from aqueous solutions by crosslinked acrylic acid/acrylamidopropane sulfonic acid hydrogels. Journal of Applied Polymer Science, 114, 1150–1159.
Akkaya, R. (2012). Synthesis and characterization of poly(2-hydroxyethylmethacrylate-hydroxyapatite) a novel composite for the removal of lead(II) from aqueous solutions. Clean-Soil, Air, Water, 40, 1257–1264.
Al, E., Güçlü, G., İyim, T. B., Emik, S., & Özgümüş, S. (2008). Synthesis and properties of starch-graft-acrylic acid/Na-montmorillonite superabsorbent nanocomposite hydrogels. Journal of Applied Polymer Science, 109, 16–22.
Arami, M., Limaee, N. Y., Mahmoodia, N. M., & Tabrizi, N. S. (2006). Equilibrium and kinetics studies for the adsorption of direct and acid dyes from aqueous solution by soy meal hull. The Journal of Hazardous Materials, B135, 171–179.
Asgher, M., & Bhatti, H. N. (2012). Removal of reactive blue 19 and reactive blue 49 textile dyes by citrus waste biomass from aqueous solution: equilibrium and kinetic study. The Canadian Journal of Chemical Engineering, 90, 412–419.
Aydın, H., & Baysal, G. (2006). Adsorption of acid dyes in aqueous solutions by shells of bittim (Pistacia khinjuk stocks). Desalination, 196, 248–259.
Bajpai, S. K., Bajpai, M., & Sharma, L. (2006). Investigation of water uptake behavior of superabsorbent polymers composed of N-vinyl 2-pyrrolidone and partially neutralized acrylic acid. Journal of Macromolecular Science, Part A. Pure and Applied Chemistry, 43, 1323–1337.
Baskaralingam, P., Pulikesi, M., Ramamurthi, V., & Sivanesan, S. (2006). Equilibrium studies for the adsorption of acid dye onto modified hectorite. Journal of Hazardous Materials, B136, 989–992.
Chowdhury, S., & Saha, P. (2011). Adsorption kinetic modeling of safranin onto rice husk biomatrix using pseudo-first- and pseudo second-order kinetic models: comparison of linear and non-linear methods. Clean-Soil, Air, Water, 39, 274–282.
Çaykara, T., & Ayçiçeği, İ. (2005). External stimuli-responsive characteristics of ionic poly[(N, N-diethylaminoethylmethacrylate)-co-(N-vinyl-2-pyrrolidone)] hydrogels. Macromolecular Materials and Engineering, 290, 468–474.
Dalaran, M., Emik, S., Güçlü, G., İyim, T. B., & Özgümüş, S. (2009). Removal of acidic dye from aqueous solutions using poly(DMAEMA-AMPS-HEMA) terpolymer/MMT nanocomposite hydrogels. Polymer Bulletin, 63, 159–171.
Dalaran, M., Emik, S., Güçlü, G., İyim, T. B., & Özgümüş, S. (2011). Study on a novel polyampholyte nanocomposite superabsorbent hydrogels: synthesis, characterization and investigation of removal of indigo carmine from aqueous solution. Desalination, 279, 170–182.
Demirbaş, E., & Nas, M. Z. (2009). Batch kinetic and equilibrium studies of adsorption of reactive blue 21 by fly ash and sepiolite. Desalination, 243, 8–21.
Erdik, E. (1993). Organik Kimyada Spektroskopik Yöntemler (Turkish). Ankara: Gazi Yayınevi. ISBN 9757373041.
Eren, E. (2010). Adsorption performance and mechanism in binding of azo dye by raw bentonite. Clean-Soil, Air, Water, 38, 758–763.
Evren, M., Acar, I., Güçlü, K., & Güçlü, G. (2013). Removal of Cu2+ and Pb2+ ions by n-vinyl 2-pyrrolidone/itaconic acid hydrogels from aqueous solutions. The Canadian Journal of Chemical Engineering. doi:10.1002/cjce.21814.
Gimbert, F., Crini, N. M., Renault, F., Badot, P. M., & Crini, G. (2008). Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. Journal of Hazardous Materials, 157(1), 34–36.
Güçlü, G., Güçlü, K., & Keleş, S. (2007). Competitive removal of nickel (II), cobalt (II) and zinc (II) ions from aqueous solutions by starch–graft–acrylic acid copolymers. Journal of Applied Polymer Science, 106, 1800–1805.
Güçlü, G. (2010). Removal of basic dyes from aqueous solutions by dimethyl terephthalate distillation residue. Desalination, 259, 53–58.
Gürses, A., Doğar, C., Yalçın, M., Açıkyıldız, M., Bayrak, R., & Karaca, S. (2006). The adsorption kinetics of the cationic dye, methylene blue, onto clay. Journal of Hazardous Materials, B131, 217–228.
Hameed, B. H., Krishni, R. R., & Sata, S. A. (2009). A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. Journal of Hazardous Materials, 162, 305–311.
Ismail, H., Irani, M., & Ahmad, Z. (2013). Starch-based hydrogels: present status and applications. International Journal of Polymeric Materials and Polymeric Biomaterials, 62, 411–420.
İyim, T. B., & Güçlü, G. (2009). Removal of basic dyes from aqueous solutions using natural clay. Desalination, 249, 1377–1379.
Jose, J., John, M., Gigimol, M. G., & Mathew, B. (2003). Synthesis, characterization, and catalytic activity of crosslinked poly(n-vinyl-2-pyrrolidone acrylic acid) copolymer–metal complexes. Journal of Applied Polymer Science, 90, 895–904.
Juang, R., Tseng, R., Wu, F., & Lee, S. J. (1997). Adsorption behaviour of reactive dyes from aqueous solutions on chitosan. Chemical Technology and Biotechnology, 70, 391–399.
Karadağ, E., Üzüm, Ö. B., & Saraydın, D. (2002). Swelling equilibria and dye adsorption studies of chemically crosslinked superabsorbent acrylamide/maleic acid hydrogels. European Polymer Journal, 38, 2133–2141.
Karim, A. B., Mounir, B., Hachkar, M., Bakasse, M., & Yaacoubi, A. (2009). Removal of basic red 46 dye from aqueous solution by adsorption onto Moroccan clay. Journal of Hazardous Materials, 168, 304–309.
Kaşgöz, H., & Durmuş, A. (2008). Dye removal by a novel hydrogel–clay nanocomposite with enhanced swelling properties. Polymers for Advanced Technologies, 19, 838–845.
Khaled, A., Nemr, A. E., El-Sikaily, A., & Abdelwahab, O. (2009). Removal of direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: adsorption isotherm and kinetic studies. Journal of Hazardous Materials, 165, 100–110.
Kim, T. H., Lee, Y., Yang, J., Lee, B., Park, C., & Kim, S. (2004). Decolorization of dye solutions by a membrane bioreactor (MBR) using white-rot fungi. Desalination, 168, 287–293.
Kim, T. H., Park, C., & Kim, S. (2005). Water recycling from desalination and purification process of reactive dye manufacturing industry by combined membrane filtration. Journal of Cleaner Production, 13, 779–786.
Kokabi, M., Sirousazar, M., & Hassan, Z. M. (2007). PVA–clay nanocomposite hydrogels for wound dressing. European Polymer Journal, 43, 773–781.
Mall, I. D., Srivastava, V. C., Agarwal, N. K., & Mishra, I. M. (2005). Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon—kinetic study and equilibrium isotherm analyses. Colloids and Surfaces, A: Physicochemical and Engineering Aspects, 264, 17–28.
McKay, G., & Ho, Y. S. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465.
Michira, I., Akinyeye, R., Baker, P., & Iwuoha, E. (2011). Synthesis and characterization of sulfonated polyanilines and application in construction of a diazinon biosensor. International Journal of Polymeric Materials, 60, 469–489.
Moghaddam, S. S., Alavi, M. R., & Arami, M. M. (2012). Response surface optimization of acid red 119 dye adsorption by mixtures of dried sewage sludge and sewage sludge ash. Clean-Soil, Air, Water, 40, 652–660.
Nagarwal, R. C., Kant, S., Singh, P. N., Maiti, P., & Pandit, J. K. (2009). Polymeric nanoparticulate system: a potential approach for ocular drug delivery. Journal of Controlled Release, 136, 2–13.
Peng, Z., & Shen, Y. (2011). Study on biological safety of polyvinyl alcohol/collagen hydrogel as tissue substitute. Polymer-Plastics Technology and Engineering, 50, 245–250.
Peppas, N. A., Bures, P., Leobandung, W., & Ichikawa, H. (2000). Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics, 50, 27–46.
Pizarro, G. C., Marambio, O. G., Jeria-Orell, M., Huerta, M. R., Rivas, B. L., & Habicher, W. D. (2008). Metal ion retention using the ultrafiltration technique: preparation, characterization of the water-soluble poly(1-vinyl-2-pyrrolidone-co-itaconic acid) and their metal complexes in aqueous solutions. Journal of Applied Polymer Science, 108, 3982–3989.
Prado, A. G. S., Torres, J. D., Faria, E. A., & Dias, S. C. L. (2004). Comparative adsorption studies of indigo carmine dye on chitin and chitosan. Journal of Colloid and Interface Science, 277, 43–47.
Rokhade, A. P., Agnihotri, S., Patil, S. A., Mallikarjuna, N. N., Kulkarni, P. V., & Aminabhavi, T. M. (2006). Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydrate Polymers, 65, 243–252.
Silverstein, R. M., & Bassler, G. C. (1966). Spectrometric identification of organic compounds. New York: John Wiley & Sons.
Şolpan, D., & Kölge, Z. (2006). Adsorption of methyl violet in aqueous solutions by poly (N-vinylpyrrolidone-co-methacrylic acid) hydrogels. Radiation Physics and Chemistry, 75, 120–128.
Thinakaran, N., Baskaralingam, P., Ravi, K. V. T., Panneerselvam, P., & Sivanesan, S. (2008). Adsorptive removal of acid blue 15: equilibrium and kinetic study. Clean-Soil, Air, Water, 36, 274–282.
Tsai, W. T., Chang, Y. M., Lui, C. W., & Lo, C. C. (2005). Adsorption of basic dyes in aqueous solution by clay adsorbent from activated bleaching earth. Applied Clay Science, 29, 149–154.
Tsai, W. T., Chang, C. Y., Ing, C. H., & Chang, C. F. (2004). Adsorption of acid dyes from aqueous solution on activated bleaching earth. Journal of Colloid and Interface Science, 275, 72–78.
Tümtürk, H., Çaykara, T., Kantoğlu, Ö., & Güven, O. (1999). Adsorption of α-amylase onto poly(N-vinyl 2-pyrrolidone/itaconic acid) hydrogels. Nuclear Instruments and Methods in Physics Research B, 151, 238–241.
Uğurlu, M., Gürses, A., & Doğar, Ç. (2007). Adsorption studies on the treatment of textile dyeing effluent by activated carbon prepared from olive stone by ZnCl2 activation. Coloration Technology, 123, 106–114.
Wang, S., Li, H., & Xu, L. (2006). Application of zeolite MCM-22 for basic dye removal from wastewater. Journal of Colloid and Interface Science, 295, 71–78.
Wang, W., & Wang, A. (2009). Preparation, characterization and properties of superabsorbent nanocomposites based on natural guar gum and modified rectorite. Carbohydrate Polymers, 77, 891–897.
Xua, S., Wang, J., Wu, R., Wang, J., & Li, H. (2006). Adsorption behaviors of acid and basic dyes on crosslinked amphoteric starch. Chemical Engineering Journal, 117, 161–167.
Zhang, L. M., Zhou, Y. J., & Wang, Y. (2006). Novel hydrogel composite for the removal of water-soluble cationic dye. Journal of Chemical Technology and Biotechnology, 81, 799–804.
Acknowledgement
This work is a masteral thesis entitled "Synthesis and Applications of N-Vinyl 2- Pyrrolidone Based Nanocomposite Hydrogels", which is prepared at Istanbul University, Institute of Science, and was supported by the Research Fund of Istanbul University, Project Number: 17262.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çöle, G., Gök, M.K. & Güçlü, G. Removal of Basic Dye from Aqueous Solutions Using a Novel Nanocomposite Hydrogel: N-Vinyl 2-Pyrrolidone/Itaconic Acid/Organo Clay. Water Air Soil Pollut 224, 1760 (2013). https://doi.org/10.1007/s11270-013-1760-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1760-5