Abstract
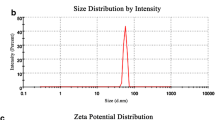
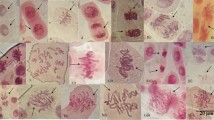
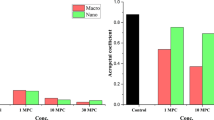
Many airborne and soil-borne nanoparticles (NPs) can enter plants, which are the primary producers in the food chain; recently, studies on the genotoxic effects of NPs on plants are emerging. In the present study, the phytotoxic and genotoxic effects of ZnO and CuO NPs on buckwheat (Fagopyrum esculentum) seedlings were estimated. The inhibition of root growth and biomass at the tested concentrations of NP suspensions and dissolved free ion suspensions were compared. Changes in root morphological features and localization of NPs inside the root epidermis cells were observed. Growth of root treated with ZnO NPs (84.9 and 89.6 %) and CuO NPs (75.4 and 80.1 %) at 2,000 and 4,000 mg L−1, respectively, was decreased significantly than control. The root morphological features and NP incorporation into the root epidermal cells at a high dose of NP showed completely different patterns compared to those for the controls. Through random amplified polymorphic DNA assays for comparison of the effect of ZnO and CuO NPs on DNA stability, it was shown as different DNA polymorphisms at 2,000 and 4,000 mg L−1 of ZnO and CuO NPs, compared to those for controls. Our results provide the first clue to the genotoxic effects of ZnO and CuO NPs on early growth of edible plants such as buckwheat.





Similar content being viewed by others
References
Angelis, K. J., Mcguffie, M., Menke, M., & Schubert, I. (2000). Adaptation to akylation damage in DNA measured by the comet assay. Environ Mol Mutagene, 36, 146–150.
An, Y. J., & Kim, M. (2009). Effect of antimony on the microbial growth and the activities of soil enzymes. Chemosphere, 74, 654–659.
Atha, D. H., Wang, H., Petersen, E. J., Cleveland, D., Holbrook, R. D., Jaruga, P., et al. (2012). Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environmental Science and Technology, 46, 1819–1827.
Atienzar, F. A., Conradi, M., Evenden, A. J., Jha, A., & Depledge, M. H. (1999). Qualitative assessment of genotoxicity using random amplified polymorphic DNA: comparison of genomic template stability with key fitness parameters in Daphnia magna to benzo[a]pyren. Environmental Toxicology and Chemistry, 18, 2275–2282.
Baek, Y. W., & An, Y. J. (2011). Microbial toxicity of metal oxide nanoparticles (Cuo, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. The Science of the Total Environment, 409, 1603–1608.
Baluska, F., Salaj, J., Mathur, J., Braun, M., Jasper, F., Samaj, J., et al. (2000). Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Developments in Biologicals, 227, 618–632.
Bibikova, T. N., Blancaflor, E. B., & Gilroy, S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. The Plant Journal, 17, 657–665.
Brunner, T. J., Wick, P., Manser, P., Spohn, P., Grass, R. N., Limbach, L. K., et al. (2006). In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environmental Science and Technology, 40, 4374–4381.
Choopun, S., Tubtimtae, A., Santhaveesuk, T., Nilphai, S., Wongrat, E., & Hongsith, N. (2009). Zinc oxide nanostructures for applications as ethanol sensors and dye sensitized solar cells. Applied Surface Science, 256, 998–1002.
Colvin, V. L. (2003). The potential environmental impact of engineered nanomaterials. Nature Biotechnology, 21, 116–1170.
Conte, C., Mutti, I., Pulish, P., Ferrarini, A., Regina, G., Maestri, E., et al. (1998). DNA fingerprinting analysis by a PCR based method for monitoring the geneotoxic effects of heavy metals pollution. Chemosphere, 37, 2739–2749.
Dietz, K. J., & Herth, S. (2011). Plant nanotoxicology. Trends in Plant Science, 16, 582–589.
Fleischer, M. A., O’Neill, R., & Ehwald, R. (1999). The pore size of non-graminaceous plant cell wall is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturon II. Plant Physiology, 121, 829–828.
Gichner, T., Žnidar, I., Wagner, E. D., & Plewa, M. J. (2009). The use of higherplants in the comet assay. In A. Dhawan & D. Anderson (Eds.), The Comet Assay in Toxicology (pp. 98–119). London: The Royal Society of Chemistry.
Grant, W. F. (1994). The present status of higher plant bioassay for the detection of environmental mutagens. Mutation Research, 310, 175–185.
Gupta, M., & Sarin, N. B. (2009). Heavy metal induced DNA changes in aquatic macrophytes: Random amplified polymorphic DNA analysis and identification. Journal of Environmental Sciences, 21, 686–690.
Kamat, P. V., & Meisel, D. (2003). Nanoscience opportunities in environmental remediation. CR Chim, 6, 999–1007.
Kedziorek, D. A., Muja, N., Walczak, P., Ruiz-Cabello, J., Gilad, A. A., Jie, C. C., et al. (2010). Gene expression profiling reveals early cellular responses to intracellular magnetic labeling with superparamagnetic iron oxide nanoparticles. Magnetic Resonance in Medicine, 63, 1031–1043.
Labra, M., Di Fabio, T., Grassi, F., Regondi, S. M., Bracale, M., Vannini, C., et al. (2003). AFLP analysis as biomarker of exposure to organic and inorganic genotoxic substances in plants. Chemosphere, 52, 1183–1188.
Lee, S. Y., Chung, H. I., Kim, S. Y., & Lee, I. S. (2012). Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environmental Science and Pollution Research, 20(2), 848–854.
Lee, W. M., An, Y. J., Yoon, H., & Kweon, H. S. (2008). Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiates) and wheat (Triticumk aestivum): plant agar test for water-insoluble nanoparticles. Environmental Toxicology and Chemistry, 27, 1915–1921.
Lin, D., & Xing, B. (2007). Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environmental Pollution, 150, 243–50.
Lin, D., & Xing, B. (2008). Root uptake and phytotoxicity of ZnO nanoparticles. Environmental Science and Technology, 42, 5580–5585.
Liu, W., Li, P. J., Qi, X. M., Zhou, Q. X., Zheng, L., Sun, T. H., et al. (2005). DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere, 61, 158–167.
López-Moreno, M. L., de la Rosa, G., Hernández-Viezcas, J. A., Castillo-Michel, H., Botez, C. E., Peralta-Videa, J. R., et al. (2010). Evidence of differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environmental Science and Technology, 44, 7315–7320.
Miller, I. S., Lynch, I., Dowling, D., Dawson, K. A., & Gallagher, W. M. (2010). Surface-induced cell signaling events control actin rearrangements and motility. Journal of Biomedical Materials Research. Part A, 93, 493–504.
Munzuroglu, O., & Geckil, H. (2002). Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Archives of Environmental Contamination and Toxicology, 43, 203–213.
Nair, R., Varghese, S. N., Nair, B. G., Maekawa, T., Yoshida, Y., & Sakthi Kumar, D. (2010). Nanoparticulate material delivery to plants. Plant Science, 179, 154–163.
Navarro, E., Baun, A., Behra, R., Hartmann, N. B., Filser, J., Miao, A. J., et al. (2008). Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology, 17(5), 372–86.
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89, 583–590.
Nel, A., Xia, T., Madler, L., & Li, N. (2006). Toxic potential of materials at the nanolevel. Science, 311, 622–627.
Nowack, B., Sculin, R., & Robinson, B. H. (2006). Critical assessment of chelant- enhanced metal phytoextraction. Journal-Erwähnungen, 40, 5225–5232.
Pisanic, T. R., 2nd, Blackwell, J. D., Shubayev, V. I., Fiñones, R. R., & Jin, S. (2007). Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials, 28, 2572–2581.
Sarkar, T., Vijay Anand, K. G., & Reddy, M. P. (2010). Effect of nickel on regulation in Jatropha curcas L. and assessment of genotoxicity using RAPD markers. BioMetals, 23, 1149–1158.
Sayes, C. M., & Warheit, D. B. (2009). Characterization of nanomaterials for toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 1, 660–670.
Scrinis, G., & Lyons, K. (2007). The emerging nano-corporate paradigm: nanotechnology and the transformation of nature, food and agri systems. Int J Sociol Agric, 15, 22–44.
Singh, N., Manshian, B., Jenkins, G. J. S., Griffiths, S. M., Williams, P. M., Maffeis, T. G., et al. (2009). Nanogenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials, 30, 3891–3914.
Shaymurat, T., Gu, J., Xu, C., Yang, Z., Zhao, Q., Liu, Y., et al. (2011). Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic (Allium sativum L.): a morphological study. Nanotoxicology, 6(3), 241–248.
Soenen, S. J., Himmerlreibh, U., Nuytten, N., Pisanic, T. R., 2nd, Ferrari, A., & De Cuyper, M. (2010). Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small, 6, 2136–2145.
Soenen, S. J., Rivera-Gil, P., Montenegro, J. M., Parak, W. J., De Smedt, S. C., & Braeckmans, K. (2011). Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today, 6, 446–465.
Steinkellner, H., Kassie, F., & Knasmuller, S. (1999). Tradescantia micronucleus assay for the assessment of the clastogenicity of Austrian water. Mutation Research, 426, 113–116.
Tamura, H., Honda, M., Sato, T., & Kamachi, H. (2005). Pb hyperaccumulation and tolerance in common buckwheat (Fagopyrim esculentum Moench). Journal of Plant Research, 118, 355–359.
US Environmental Protection Agency (1996) Ecological effects test guidelines. OPPTS 850. 4200. Seed germination root elongation toxicity test. US Environmental Protection Agency, Washington.
Wang, Z. L. (2004). Zinc oxide nanostructures: growth, properties and applications. Journal of Physics: Condensed Matter, 16, R829–R858.
Weinstein, L. H., Laurence, J. A., Mandle, R. H., & Wälti, K. (1990). Use of native and cultivated plants as bioindicators and biomonitors of pollution damage. In W. Wang, W. Gorsuch, & W. R. Lower (Eds.), Plants of toxicity assessment: ASTM STP1091 (pp. 117–126). Philadelphia: ASTM International.
Wolf, H. D., Blust, R., & Backeljau, T. (2004). The use of RAPD in ecotoxicology. Mutation Research, 566, 249–262.
Wu, X., Tan, Y., Mao, H., & Zhang, M. (2010). Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. International Journal of Nanomedicine, 5, 385–399.
Zhu, H., Han, J., Xiao, J. Q., & Jin, Y. (2008). Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. Journal of Environmental Monitoring, 10, 713–717.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010–0021911). This study was supported by post-doctoral researchers support program of Ewha Woman’s University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 490 kb)
Rights and permissions
About this article
Cite this article
Lee, S., Chung, H., Kim, S. et al. The Genotoxic Effect of ZnO and CuO Nanoparticles on Early Growth of Buckwheat, Fagopyrum Esculentum . Water Air Soil Pollut 224, 1668 (2013). https://doi.org/10.1007/s11270-013-1668-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1668-0