Abstract
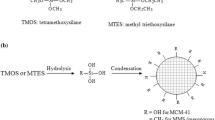
Poised to gain insight into nitrate adsorption and removal processes from water through employment of modified surfaces, a well-defined inorganic manganese species was used in connection with hydrophobic mesoporous silica. To this end, the surface of hydrophobic mesoporous silica was modified by coating silica with a manganese oxychloride (Mn8O10Cl3) nanoparticle layer. A sol–gel method was utilized for the synthesis of hydrophobic silica, using tetraethyl orthosilicate–methyl triethoxysilane (TEOS–MTES) as precursors. Subsequent coating with Mn8O10Cl3 took place by mixing MnCl2 and NaOH with hydrophobic silica. Physicochemical characterization of the Mn8O10Cl3-coated silica was carried out by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and N2 sorption. The achieved surface modification reduced remarkably the specific surface area by 80.7 % and influenced the ability of nitrates to adsorb on Mn-modified silica. Nitrate adsorption kinetics on Mn8O10Cl3-coated silica was studied by a batch reactor. Process parameters including pH, temperature, and initial nitrate concentration were examined thoroughly. The experimental adsorption data were fitted satisfactorily through Langmuir isotherm equations and were found to be well-represented by a pseudo-second-order kinetic model. The collective data emphasize the significance of well-defined inorganic manganese phases, coating hydrophobic silica, in optimally influencing water decontamination from pollutant nitrates.










Similar content being viewed by others
References
Akosman, C., & Özdemir, T. (2010). Adsorption dynamics and equilibrium studies of nitrate onto various soils. Fresenious Enviromental Bulletin, 19(10), 2246–2252.
Bentrup, U., Brückner, A., Richter, M., & Fricke, R. (2001). NO x adsorption on MnO2/NaY composite: an in situ FTIR and EPR study. Applied Catalysis B: Environment, 32, 229–241.
Buisson, G. (1976). Préparation et propriétes d'un oxychlorure de manganese de formule Mn8O10Cl3. Journal of Solid State Chemistry, 19(2), 175–178.
Catts, J. G., & Langmuir, D. (1986). Adsorption of Cu, Pb and Zn by δMnO2: applicability of the site binding-surface complexation model. Applied Geochemistry, 1, 255–264.
Chabani, M., Amrane, A., & Bensmaili, A. (2007). Kinetics of nitrates adsorption on Amberlite IRA 400 resin. Desalination, 206, 560–567.
Deguin, A. (1988). Incidence des resines echangeuses d'anions sur la qualite de l'eau traitee selon le procede nitracycle. L'Eau, 4, 213–234.
Euzen, P., Leone, P., Palvadeau, P., Queignec, M., & Rouxel, J. (1992). Synthesis and structural studies of manganese oxyhalides with a multisite framework. Materials Research Bulletin, 27(11), 1295–1300.
Euzen, P., Leone, P., Palvadeau, P., Queignec, M., & Rouxel, J. (1997). Investigations by magnetic measurements and neutron diffraction studies of the magnetic ordering of Mn8O10Cl3. Journal of Magnetism and Magnetic Materials, 172, 153–164.
Gadde, R. R., & Laitinen, H. A. (1974). Heavy metal adsorption by hydrous iron and manganese oxides. Analytical Chemistry, 46(13), 2022–2026.
Gao, T., Fjeiivag, H., & Norby, P. (2009). A comparison study on Raman scattering properties of alpha- and beta-MnO2. Analytica Chimica Acta, 648, 235–239.
Hench, L. L., & West, J. K. (1990). The sol–gel process. Chemical Reviews, 90, 33–72.
Islam, M., & Patel, R. (2009). Nitrate sorption by thermally activated Mg/Al chloride hydrotalcite-like compound. Journal of Hazardous Materials, 169, 524–531.
Jaafari, K., Elmaleh, S., Coma, J., & Benkhouja, K. (2001). Equilibrium and kinetics of nitrate removal by protonated cross-linked chitosan. Water SA, 27(1), 9–13.
Julien, C.M., & Massot, M. (2004). Vibrational spectroscopy of electrode materials for rechargeable lithium batteries III. Oxide frameworks, Proceedings of the International Workshop “Advanced techniques for energy sources investigation and testing”: Sofia, Bulgaria (L3. pp. 1-17).
Kawano, Y., Denofre, S., & Gushikem, Y. (1994). Raman and infrared spectra of silica gel and niobium(V) oxide grafted on silica gel surface and their dependence on pretreatment temperatures. Vibrational Spectroscopy, 7, 293–302.
Kingma, K. J., & Hemley, R. J. (1994). Raman spectroscopic study of microcrystalline silica. American Mineralogist, 79, 269–273.
McKay, G., Blair, H. S., & Gardener, J. R. (1982). Adsorption of dyes on chitin. I. Equilibrium studies. Journal of Applied Polymer Science, 27, 3043–3057.
Mironova-Ulmane, N., Kuzmin, A., & Grube, M. (2009). Raman and infrared spectromicroscopy of manganese oxides. Journal of Alloys and Compounds, 480, 97–99.
Murray, J. W. (1974). The surface chemistry of hydrous manganese dioxide. Journal of Colloid and Interface Science, 46(3), 357–371.
Noons, R. E., Devonshire, R., Clapp, T. V., Ojha, S. M., & McCarthy, O. (2008). Analysis of waveguide silica glasses using Raman microscopy. Journal of Non-Crystalline Solids, 354, 3059–3071.
Öztürk, N., & Bektas, T. E. (2004). Nitrate removal from aqueous solution by adsorption onto various materials. Journal of Hazardous Materials, B112, 155–162.
Richard, Y., & Leprince, A. (1980). L'azote dans le traitement des eaux potables—Les traitements biologiques. L'eau, 4, 167–181.
Richard, Y., Leprince, A., Martin, G., & Leblane, C. (1980). Denitrification of water for human consumption. Progress in Water Technology, 12, 173–191.
Stöber, W., Fink, A., & Bohn, E. (1966). Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Science, 26, 62–69.
Strebel, O., Duynisved, W. H. M., & Boettcher, J. (1989). Nitrate pollution of groundwater in Western Europe. Agriculture, Ecosystems & Environment, 26, 189–214.
Su, Q., Pan, B., Wana, S., Zhang, W., & Lv, L. (2010). Use of hydrous manganese dioxide as a potential sorbent for selective removal of lead, cadmium, and zinc ions from water. Journal of Colloid and Interface Science, 349, 607–612.
Toussaint, G., Rodriguez, M. A., Cloots, R., Rubio, J., Rubio, F., Vertruyen, B., & Henrist, C. (2011). Characterization of surface and porous properties of synthetic hybrid lamellar silica. Journal of Non-Crystalline Solids, 357, 951–957.
Weber, T. W., & Chakraborty, R. K. (1974). Pore and solid diffusion models for fixed bed adsorbents. American Institute of Chemical Engineers Journal, 20, 228–238.
White, W. B., & Keramidas, V. G. (1972). Vibrational spectra of oxides with the C-type rare earth oxide structure. Spectrochimica Acta, 28A, 501–509.
Yang, H., Pi, P., Cai, Z.-Q., Wen, X., Wang, X., Cheng, J., & Yang, Z.-R. (2010). Facile preparation of super-hydrophobic and super-oleophilic silica film on stainless steel mesh via sol–gel process. Applied Surface Science, 256, 4095–4102.
Zheng, Y., & Wang, A. (2010). Nitrate adsorption using poly(dimethyl diallyl ammonium chloride)/polyacrylamide hydrogel. Journal of Chemical and Engineering Data, 55, 3494–3500.
Acknowledgments
This work was cofinanced by the EU–ESF and Greek national funds through the NSRF-Heracleitus II program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 168 kb)
Rights and permissions
About this article
Cite this article
Halevas, E., Malakopoulos, A., Delimitis, A. et al. Manganese Oxychloride-Modified Hydrophobic Silica Targets Removal of Nitrates from Water. Water Air Soil Pollut 224, 1598 (2013). https://doi.org/10.1007/s11270-013-1598-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1598-x