Abstract
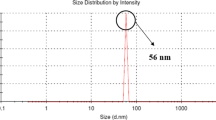
High concentration of fluoride in groundwater is a longstanding health problem. Fluoride adsorption capacity of termite mound (TM), containing mainly silicon, aluminum, iron, and titanium oxides, was investigated under a batch adsorption system. The influence of parameters such as contact time, solution pH, adsorbent dose, initial fluoride concentration, and the presence of competing anions was investigated. Equilibrium was achieved within 10 min of agitation time. A high percentage (~90 %) of fluoride removal was obtained in a wide pH range 3–8, which is important in the practical application. Kinetics data followed the pseudo-second-order model (R 2 > 0.99). The Dubinin–Radushkevich isotherm described most satisfactorily (R 2 = 0.968, χ 2 = 0.09) the equilibrium adsorption, giving a sorption capacity of 2.70 mg/g. The obtained mean free energy (E DR = 11.62 kJ/mol) suggested that chemisorption should be mainly responsible for fluoride adsorption. Fluoride removal was significantly decreased in the presence of carbonate and phosphate ions, whereas slightly increased in the presence of chloride, nitrate, and sulfate. The adsorbent reduced 7.56 mg/L fluoride content of groundwater to below 1.5 mg/L. The fluoride-loaded TM was successfully regenerated using calcined eggshell or NaOH solution with insignificant loss of metals. The adsorption efficiency of the regenerated TM was comparable to the fresh TM. The results obtained from this study could provide important information for evaluating the application of TM for defluoridation.










Similar content being viewed by others
References
Abdulrahman, A. (1990). Foraging activity and control of termites in western Ethiopia. PhD thesis. London: University of London.
Abdus-Salam, N., & Itiola, A. D. (2012). Potential application of termite mound for adsorption and removal of Pb(II) from aqueous solutions. Journal of the Iranian Chemical Society, 9, 373–382.
Appel, C., & Ma, L. Q. (2002). Concentration, pH, and surface charge effects on Cd and Pb sorption in three tropical soils. Journal of Environmental Quality, 31, 581–589.
Appel, C., Lena, Q., Ma, R., Rhue, D., & Kennelle, E. (2003). Point of zero charge determination in soils and minerals via traditional methods and detection of electroacoustic mobility. Geoderma, 113, 77–97.
Ayoob, S., & Gupta, A. K. (2009). Performance evaluation of alumina cement granules in removing fluoride from natural and synthetic waters. Chemical Engineering Journal, 150, 485–491.
Balázsi, C., Kövér, Z., Horváth, E., Németh, C., Kasztosky, Z., Kurunczi, S., et al. (2007). Examination of calcium-phosphates prepared from eggshell. Material Science Forum, 537–538, 105–112.
Bhatnagar, A., Kumar, E., & Sillanpää, M. (2011). Fluoride removal from water by adsorption—a review. Chemical Engineering Journal, 171, 811–840.
Biswas, K., Gupta, K., & Ghosh, U. C. (2009). Adsorption of fluoride by hydrous iron(III)-tin(IV) bimetal mixed oxide from the aqueous solutions. Chemical Engineering Journal, 149, 196–206.
Brown, G. E., Henrich, V. E., Casey, W. H., Clark, D. L., Eggleston, C., Felmy, A., et al. (1998). Metal oxide surfaces and their interactions with aqueous solutions and microbial organisms. Chemical Reviews, 99, 77–174.
Camacho, L. M., Torres, A., Saha, D., & Deng, S. (2010). Adsorption equilibrium and kinetics of fluoride on sol–gel-derived activated alumina adsorbents. Journal of Colloid and Interface Science, 349, 307–313.
Chauhan, V. S., Dwivedi, P. K., & Iyengar, L. (2007). Investigations on activated alumina based domestic defluoridation units. Journal of Hazardous Materials, 139, 103–107.
Chen, N., Zhang, Z., Feng, C., Li, M., Zhu, D., Chen, R., et al. (2010a). An excellent fluoride sorption behavior of ceramic adsorbent. Journal of Hazardous Materials, 183, 460–465.
Chen, N., Zhang, Z., Feng, C., Sugiura, N., Li, M., & Chen, R. (2010b). Fluoride removal from water by granular ceramic adsorption. Journal of Colloid and Interface Science, 348, 579–784.
Chen, N., Zhang, Z., Feng, C., Li, M., Zhu, D., & Sugiura, N. (2011a). Studies on fluoride adsorption of iron-impregnated granular ceramics from aqueous solution. Materials Chemistry and Physics, 125, 293–298.
Chen, N., Zhang, Z., Feng, C., Zhu, D., Yang, Y., & Sugiura, N. (2011b). Preparation and characterization of porous granular ceramic containing dispersed aluminum and iron oxides as adsorbents for fluoride removal from aqueous solution. Journal of Hazardous Materials, 186, 863–868.
Cornell, R. M., & Schwertmann, U. (1996). The iron oxides: structure, properties, reactions, occurrence and uses. New York: VCH.
Daifullah, A. A. M., Yakout, S. M., & Elreefy, S. A. (2007). Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from stem pyrolysis of rich straw. Journal of Hazardous Materials, 147, 633–643.
Das, N., Pattanaik, P., & Das, R. (2005). Defluoridation of drinking water using activated titanium rich bauxite. Journal of Colloid and Interface Science, 292, 1–10.
DIN ISO 13878. (1998). Bodenbeschaffenheit - Bestimmung des Gesamt-Stickstoffs durch trockene Verbrennung (Elementaranalyse). Berlin: Beuth.
DIN ISO 10 694. (1996). Bodenbeschaffenheit-Bestimmung von organischem Kohlenstoff und Gesamtkohlenstoff nach trockener Verbrennung (Elementaranalyse). Berlin: Beuth.
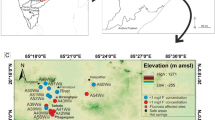
Furi, W., Razack, M., Abiye, T. A., Ayenew, T., & Legesse, D. (2011). Fluoride enrichment mechanism and geospatial distribution in the volcanic aquifers of the Middle Awash basin, Northern Main Ethiopian Rift. Journal of African Earth Sciences, 60, 315–327.
Gergely, G., Wéber, F., Lukács, I., Tóth, A. L., Horváth, Z. E., Mihály, J., et al. (2010). Preparation and characterization of hydroxyapatite from eggshell. Ceramics International, 36, 803–806.
Goldberg, S., & Johnstony, C. T. (2001). Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. Journal of Colloid and Interface Science, 234, 204–216.
Guo, H., Stüben, D., & Berner, Z. (2007). Removal of arsenic from aqueous solution by natural siderite and hematite. Applied Geochemistry, 22, 1039–1051.
Hayes, K. F., Papelis, C., & Leckie, J. O. (1988). Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interface. Journal of Colloid and Interface Science, 125, 717–726.
Ho, Y. S., & McKay, G. (1999). Pseudo-second-order model for sorption process. Process Biochemistry, 34, 451–465.
Jagtap, S., Thakre, D., Wanjari, S., Kamble, S., Labhsetwar, N., & Rayalu, S. (2009). New modified chitosan-based adsorbent for defluoridation of water. Journal of Colloid and Interface Science, 332, 280–290.
Jagtap, S., Yenkie, M. K., Labhsetwar, N., & Rayalu, S. (2012). Fluoride in drinking water and defluoridation of water. Chemical Reviews, 112, 2454–2466.
Jouquet, P., Mamou, P., Lepage, M., & Velde, B. E. (2002). Effect of termite on clay minerals in tropical soils: fungus-growing termites as weathering agents. European Journal of Soil Science, 53, 521–527.
Kaufhold, S., Dohrmann, R., Abidin, Z., Henmi, T., Matsue, N., Eichinger, L., et al. (2010). Allophane compared with other sorbent minerals for the removal of fluoride from water with particular focus on a mineable Ecuadorian allophane. Applied Clay Science, 50, 23–33.
Kumar, E., Bhatnagar, A., Ji, M., Jung, W., Lee, S.-H., Kim, S.-J., et al. (2009). Defluoridation from aqueous solutions by granular ferric hydroxide (GFH). Water Research, 43, 490–498.
Liu, C., & Evett, J. B. (2003). Soil properties—testing, measurement, and evaluation. ISBN: 0-13-093005-9. USA: Banta Book Company.
Liu, Q., Guo, H., & Shan, Y. (2010). Adsorption of fluoride on synthetic siderite from aqueous solution. Journal of Fluorine Chemistry, 131, 635–641.
Lopez-Hernandez, D., Brossard, M., Fardeau, J. C., & Lepage, M. (2006). Effect of different termite feeding groups on P sorption and P availability in African and South American savannas. Biology and Fertility of Soils, 42, 207–214.
Lucy, M. C., Arely, T., Dipendu, S., & Shuguang, D. (2010). Adsorption equilibrium and kinetics of fluoride on sol–gel-derived activated alumina adsorbents. Journal of Colloid and Interface Science, 349, 307–313.
Mahmood, T., Din, S. U., Naeem, A., Mustafa, S., Waseem, M., & Hamayun, M. (2012). Adsorption of arsenate from aqueous solution on binary mixed oxide of iron and silicon. Chemical Engineering Journal, 192, 90–98.
Maiti, A., Basu, J. K., & De, S. (2011). Chemical treated laterite as promising fluoride adsorbent for aqueous system and kinetic modeling. Desalination, 265, 28–36.
Maliyekkal, S. M., Shukla, S., Philip, L., & Nambi, I. M. (2008). Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules. Chemical Engineering Journal, 140, 183–192.
McBride, M. B. (1997). A critique of diffuse double layer models applied to colloid and surface chemistry. Clays and Clay Minerals, 45, 598–608.
Meenakshi, S., Sundaram, C. S., & Sukumar, R. (2008). Enhanced fluoride sorption by mechanochemically activated kaolinites. Journal of Hazardous Materials, 153, 164–172.
Mohapatra, M. S., Anand, S., Mishra, B. K., Giles, D. E., & Singh, P. (2009). Review of fluoride removal from drinking water. Journal of Environmental Management, 91, 67–77.
Nigussie, W., Zewge, F., & Chandravanshi, B. S. (2007). Removal of excess fluoride from water using waste residue from alum manufacturing process. Journal of Hazardous Materials, 147, 954–963.
OADB (2001). Phase II: Integrated pilot project for termite control in 8 districts of East and West Wellega Zone, Oromia. Finfinnee: Oromia Agricultural Development Bureau (OADB).
Ramos, L. R., Utrilla, R. J., Castillo, N. M., & Polo, M. S. (2010). Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chemical Engineering Journal, 158, 458–467.
Rango, T., Bianchini, G., Beccaluva, L., & Tassinari, R. (2010). Geochemistry and water quality assessment of central Main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. Journal of African Earth Sciences, 57, 479–491.
Sakhare, N., Lunge, S., Rayalu, S., Bakardjiva, S., Subrt, J., Devotta, S., et al. (2012). Defluoridation of water using calcium aluminate material. Chemical Engineering Journal, 203, 406–414.
Sarı, A., & Tuzen, M. (2009). Biosorption of As(III) and As(V) from aqueous solution by macrofungus (Inonotus hispidus) biomass: equilibrium and kinetic studies. Journal of Hazardous Materials, 164, 1372–1378.
Sarkar, M., Banerjee, A., Pramanick, P. P., & Sarkar, A. R. (2006). Use of laterite for the removal of fluoride from contaminated drinking water. Journal of Colloid and Interface Sciences, 302, 432–441.
Semhi, K., Chaudhuri, S., Claurer, N., & Boeglin, J. L. (2008). Impact of termite activity on soil environment: a perspective from their soluble chemical components. International journal of Environmental Science and Technology, 5, 431–444.
Shriver, D. F., Atkins, P. W., & Langford, C. H. (1994). Inorganic chemistry. Oxford: Oxford University Press.
Solangi, I. B., Memon, S., & Bhanger, M. I. (2009). Removal of fluoride from aqueous environment by modified Amberlite resin. Journal of Hazardous Materials, 171, 815–819.
Sujana, M. G., & Anand, S. (2010). Iron and aluminium based mixed hydroxides: a novel sorbent for fluoride removal from aqueous solutions. Applied Surface Science, 256, 6956–6962.
Sujana, M. G., Thakur, R. S., & Rao, S. B. (1998). Removal of fluoride from aqueous solution by alum sludge. Journal of Colloid and Interface Science, 206, 94–101.
Tadesse, S., Milesi, J.-P., & Deschamps, Y. (2003). Geology and mineral potential of Ethiopia: a note on geology and mineral map of Ethiopia. Journal of African Earth Sciences, 36, 273–313.
Thakre, D., Rayalu, S., Kawade, R., Meshram, S., Subrt, J., & Labhsetwar, N. (2010). Magnesium incorporated bentonite clay for defluoridation of drinking water. Journal of Hazardous Materials, 180, 122–130.
Thole, B. (2011). Defluoridation kinetics of 200 °C calcined bauxite, gypsum, and magnesite and breakthrough characteristics of their composite filter. Journal of Fluorine Chemistry, 132, 529–535.
Thongthai, W. (2011). Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent. Ceramics International, 37, 3291–3298.
Tian, Y., Wu, M., Liu, R., Wang, D., Lin, X., Liu, W., et al. (2011). Modified native cellulose fibers: a novel efficient adsorbent for both fluoride and arsenic. Journal of Hazardous Materials, 185, 93–100.
Tilahun, A., Kebede, F., Yamoah, C., Erens, H., Mujinya, B. B., Verdoodt, A., et al. (2012). Quantifying the masses of Macrotermes subhyalinus mounds and evaluating their use as a soil amendment. Agriculture, Ecosystems and Environment, 157, 54–59.
Tor, A., Danaoglu, N., Arslan, G., & Cengeloglu, Y. (2009). Removal of fluoride from water by using granular red mud: batch and column studies. Journal of Hazardous Materials, 164, 271–278.
Tripathy, S. S., & Raichur, A. M. (2008). Abatement of fluoride from water using manganese dioxide-coated activated alumina. Journal of Hazardous Materials, 153, 1043–1051.
Wang, Y., & Reardon, E. J. (2001). Activation and regeneration of a soil sorbent for defluoridation of drinking water. Applied Geochemistry, 16, 531–539.
WHO. (2006). Guidelines for drinking water quality. First addendum to 3rd edition. Vol. 1: Recommendations. Geneva: World Health Organization.
Zhao, Y., Li, X., Liu, L., & Chen, F. (2008). Fluoride removal by Fe(III)-loaded ligand exchange cotton cellulose adsorbent from drinking water. Carbohydrate Polymers, 272, 144–150.
Acknowledgments
The first author is thankful to the Ethiopian Engineering Capacity Building Program (ECBP) and the German Academic Exchange Service (DAAD) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fufa, F., Alemayehu, E. & Lennartz, B. Defluoridation of Groundwater Using Termite Mound. Water Air Soil Pollut 224, 1552 (2013). https://doi.org/10.1007/s11270-013-1552-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1552-y