Abstract
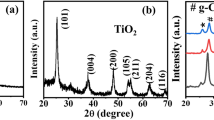
In this work, photocatalytic degradation of two reactive dyes, Reactive Yellow 84 (RY 84) and Reactive Black 5 (RB 5), on FeTiO3/TiO2 heterojunction in the presence of UV–visible radiation and H2O2 has been reported. FeTiO3/TiO2 heterojunction has been prepared from ilmenite FeTiO3 and anatase TiO2 by employing oxalic acid as an organic linker. FeTiO3/TiO2 ratios have been varied from 1 to 5 wt.%, and the materials were characterized by X-ray diffraction, scanning electron microscope and diffused reflectance UV–visible spectroscopic analysis. The photocatalytic activity of FeTiO3/TiO2 heterojunction for the degradation of the organic dyes RY 84 and RB 5 in the presence of UV–visible light was found to be higher than that of pure TiO2. The addition of H2O2 increases the rate of degradation of both dyes on FeTiO3/TiO2 heterojunction. It facilitates the fast degradation of dye solutions even when their concentration was above 100 mg/l, which is otherwise very slow due to the low transmittance of light by the dye solution. The extent of mineralisation of the reactive dye during photocatalytic degradation was estimated from chemical oxygen demand analysis. FeTiO3/TiO2 heterojunction photocatalyst was also found to have good photostability; the material retains almost 97 % of its initial activity even in the fifth cycle.












Similar content being viewed by others
References
Arabatzis, I. M., Stergiopoulos, T., Bernard, M. C., Labou, D., Neophytides, S. G., & Falaras, P. (2003). Silver-modified titanium dioxide thin films for efficient photodegradation of methyl orange. Applied Catalysis B: Environmental, 42, 187–201.
Bessekhouad, Y., Robert, D., & Weber, J. V. (2004). Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. Journal of Photochemistry and Photobiology A: Chemistry, 163, 569–580.
Bessekhouad, Y., Chaoui, N., Trzpit, M., Ghazzal, N., Robert, D., & Weber, J. V. (2006). UV–Vis versus visible degradation of Acid Orange II in a coupled CdS/TiO2 semiconductors suspension. Journal of Photochemistry and Photobiology A: Chemistry, 183, 218–224.
Bhatnagar, A., & Minocha, A. K. (2006). Conventional and non-conventional adsorbents for removal of pollutants from water—a review. Indian Journal of Chemical Technology, 13, 203–217.
Bozzi, A., Yuranova, T., Mielczarski, J., Lopez, A., & Kiwi, J. (2002). Abatement of oxalates catalyzed by Fe-silica structured surfaces via cyclic carboxylate intermediates in photo-Fenton reactions. Chemical Communications, 2202–2203.
Bozzi, A., Yuranova, T., Mielczarski, J., & Kiwi, J. (2004). Evidence for immobilized photo-Fenton degradation of organic compounds on structured silica surfaces involving Fe recycling. New Journal of Chemistry, 28, 519–526.
Chai, S. Y., Kim, Y. J., & Lee, W. I. (2006). Photocatalytic WO3/TiO2 nanoparticles working under visible light. Journal of Electroceramics, 17, 909–912.
Crini, G. (2006). Non-conventional low-cost adsorbents for dye removal: a review. Bioresource Technology, 97, 1061–1085.
Gao, B., Kim, Y. J., Chakraborty, A. K., & Lee, W. I. (2008). Efficient decomposition of organic compounds with FeTiO3/TiO2 heterojunction under visible light irradiation. Applied Catalysis B: Environmental, 83, 202–207.
Goswami, D. Y. (1997). A review of engineering developments of aqueous phase solar photocatalytic detoxification and disinfection processes. Journal of Solar Energy Engineering, 119, 101–107.
Gupta, V. K., Jain, R., & Varshney, S. (2007). Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk—an agricultural waste. Journal of Hazardous Materials, 142, 443–448.
Hâncu, D., Green, J., & Beckman, E. J. (2002). H2O2 in CO2: sustainable production and green reactions. Accounts of Chemical Research, 35, 757–764.
He, D., & Lin, F. (2007). Preparation and photocatalytic activity of anatase TiO2 nanocrystallites with high thermal stability. Materials Letters, 61, 3385–3387.
He, J., Tao, X., Ma, W., & Zhao, J. (2002). Heterogeneous photo-Fenton degradation of an azo dye in aqueous H2O2/iron oxide dispersions at neutral pHs. Chemistry Letters, 1, 86–87.
Ho, W., & Yu, J. C. (2006). Sonochemical synthesis and visible light photocatalytic behavior of CdSe and CdSe/TiO2 nanoparticles. Journal of Molecular Catalysis A: Chemical, 247, 268–274.
Ho, W., Yu, J. C., Lin, J., Yu, J., & Li, P. (2004). Preparation and photocatalytic behavior of MoS2 and WS2 nanocluster sensitized TiO2. Langmuir, 20, 5865–5869.
Kang, M. G., Han, H.-E., & Kim, K.-J. (1999). Enhanced photodecomposition of 4-chlorophenol in aqueous solution by deposition of CdS on TiO2. Journal of Photochemistry and Photobiology A: Chemistry, 125, 119–125.
Kennedy, J. H., & Frese, K. W. (1978). Photooxidation of water at α–Fe2 O3 electrodes. Journal of the Electrochemical Society, 125, 709–714.
Kim, Y. J., Gao, B., Han, S. Y., Jung, M. H., Chakraborty, A. K., Ko, T., et al. (2009). Heterojunction of FeTiO3 nanodisc and TiO2 nanoparticle for a novel visible light photocatalyst. Journal of Physical Chemistry C, 113, 19179–19184.
Konstantinou, I. K., & Albanis, T. A. (2003). Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways. Applied Catalysis B: Environmental, 42, 319–335.
Kormann, C., Bahnemann, D. W., & Hoffmann, M. R. (1988). Preparation and characterization of quantum-size titanium dioxide. Journal of Physical Chemistry, 92, 5196–5201.
Kumar, A., & Jain, A. K. (2001). Photophysics and photochemistry of colloidal CdS-TiO2 coupled semiconductors—photocatalytic oxidation of indole. Journal of Molecular Catalysis A: Chemical, 165, 265–273.
Linsebigler, A. L., Lu, G., & Yates, J. T., Jr. (1995). Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chemical Reviews, 95, 735–758.
Liu, J., Yang, R., & Li, S. (2006). Preparation and characterization of the TiO2-V2O5 photocatalyst with visible-light activity. Rare Metals, 25, 636–642.
Lo, S.-C., Lin, C.-F., Wu, C.-H., & Hsieh, P.-H. (2004). Capability of coupled CdSe/TiO2 for photocatalytic degradation of 4-chlorophenol. Journal of Hazardous Materials, 114, 183–190.
Mittal, A., Kurup, L., & Gupta, V. K. (2005). Use of waste materials—bottom ash and de-oiled soya, as potential adsorbents for the removal of Amaranth from aqueous solutions. Journal of Hazardous Materials, 117, 171–178.
Morikawa, T., Ohwaki, T., Suzuki, K.-I., Moribe, S., & Tero-Kubota, S. (2008). Visible-light-induced photocatalytic oxidation of carboxylic acids and aldehydes over N-doped TiO2 loaded with Fe, Cu or Pt. Applied Catalysis B: Environmental, 83, 56–62.
Murov, S. L., Hug, G. L., & Carmichael, I. (1993). Handbook of photochemistry (2nd ed.). New York: M. Dekker.
Noorjahan, M., Durga Kumari, V., Subrahmanyam, M., & Panda, L. (2005). Immobilized Fe(III)-HY: an efficient and stable photo-Fenton catalyst. Applied Catalysis B: Environmental, 57, 291–298.
Pagga, U., & Brown, D. (1986). The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere, 15, 479–491.
Pal, B., Sharon, M., & Nogami, G. (1999). Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Materials Chemistry and Physics, 59, 254–261.
Pendlebury, S. R., Barroso, M., Cowan, A. J., Sivula, K., Tang, J., Graetzel, M., et al. (2011). Dynamics of photogenerated holes in nanocrystalline α-Fe2O3 electrodes for water oxidation probed by transient absorption spectroscopy. Chemical Communications, 47, 716–718.
Robinson, T., McMullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77, 247–255.
Saquib, M., & Muneer, M. (2003). TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dyes and Pigments, 56, 37–49.
Scherrer, P. (1918). Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Göttingen Nachrichten, 2, 98–100.
So, C. M., Cheng, M. Y., Yu, J. C., & Wong, P. K. (2002). Degradation of azo dye Procion Red MX-5B by photocatalytic oxidation. Chemosphere, 46, 905–912.
Sobana, N., Muruganadham, M., & Swaminathan, M. (2006). Nano-Ag particles doped TiO2 for efficient photodegradation of direct azo dyes. Journal of Molecular Catalysis A: Chemical, 258, 124–132.
Solozhenko, E. G., Soboleva, N. M., & Goncharuk, V. V. (1995). Decolourization of azodye solutions by Fenton's oxidation. Water Research, 29, 2206–2210.
Song, H., Jiang, H., Liu, X., & Meng, G. (2006). Efficient degradation of organic pollutant with WOx modified nano TiO2 under visible irradiation. Journal of Photochemistry and Photobiology A: Chemistry, 181, 421–428.
Tristão, J. C., Magalhães, F., Corio, P., & Sansiviero, M. T. C. (2006). Electronic characterization and photocatalytic properties of CdS/TiO2 semiconductor composite. Journal of Photochemistry and Photobiology A: Chemistry, 181, 152–157.
Yin, H., Wada, Y., Kitamura, T., Sakata, T., Mori, H., & Yanagida, S. (2001). Enhanced photocatalytic dechlorination of 1,2,3,4-tetrachlorobenzene using nanosized CdS/TiO2 hybrid photocatalyst under visible light irradiation. Chemistry Letters, 4, 334–335.
Zhang, F., Yediler, A., Liang, X., & Kettrup, A. (2004). Effects of dye additives on the ozonation process and oxidation by-products: a comparative study using hydrolyzed C.I. Reactive Red 120. Dyes and Pigments, 60, 1–7.
Zhang, N., Liu, S., & Xu, Y. J. (2012). Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale, 4, 2227–2238.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivakumar, S., Selvaraj, A., Ramasamy, A.K. et al. Enhanced Photocatalytic Degradation of Reactive Dyes over FeTiO3/TiO2 Heterojunction in the Presence of H2O2 . Water Air Soil Pollut 224, 1529 (2013). https://doi.org/10.1007/s11270-013-1529-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1529-x