Abstract
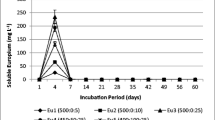
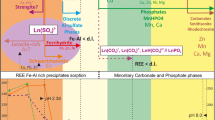
Acid mine drainage (AMD) generated by some coal mines in New Zealand is currently treated by the addition of alkaline reagents which neutralize acidity, triggering the precipitation of dissolved metals as insoluble hydroxides. Some trace metals (Ni, Zn, Cu, Cd, and Pb) are discharged into receiving water bodies due to incomplete hydroxide precipitation at circum-neutral pH. This study investigated the incorporation of lignite-derived humic substances (HS) for metal complexation and removal during AMD treatment by Ca(OH)2 and CaCO3 neutralization. For Ca(OH)2 neutralization, addition of HS (regardless of dosing sequence) enhanced the removal of Zn, Cu, and Cd, probably due to the incorporation of metal–humate complex into settling flocs (via aggregation, co-precipitation, and adsorption) that were subsequently removed by sedimentation. However, additional removal of Ni and Pb was statistically indeterminate, which was ascribed to the low complexation affinity of Ni and high removal of Pb by adsorption onto Fe/Al hydroxides. Conversely, for CaCO3 neutralization, addition of HS only marginally enhanced Cd removal, with the removal of metals probably dominated by adsorption onto the abundant undissolved calcite. Equilibrium speciation modelling showed that about 25% and 38% of the remaining Cu and Pb in the treated AMD were complexed with HS, while only 5% of remaining Cd and less than 1 wt% of remaining Ni and Zn were organically complexed. In the AMD-receiving water bodies, about 20 mg l−1 of HS would be required for complete complexation (>95%) of Cu and Pb and 50 mg l−1 for Cd, whereas Zn and Ni complexation would not occur at natural stream HS concentrations.





Similar content being viewed by others
References
Al-Reasi, H. A., Wood, C. M., & Smith, D. S. (2011). Physicochemical and spectroscopic properties of natural organic matter (NOM) from various sources and implications for ameliorative effects on metal toxicity to aquatic biota. Aquatic Toxicology, 103(3–4), 179–190.
American Society for Testing and Materials (ASTM) (2008). Standard Practice for Coagulation-Flocculation Jar Test of Water. West Conshohocken, PA. D 2035.
Bogush, A. A., & Voronin, V. G. (2011). Application of a peat-humic agent for treatment of acid mine drainage. Mine Water and the Environment, 30, 185–190.
Cezikova, J., Kozler, J., Madronová, L., Novák, J. R., & Janoš, P. (2001). Humic acids from coals of the North-Bohemian coal field: II Metal-binding capacity under static conditions. Reactive and Functional Polymers, 47(2), 111–118.
Chen, J., Gu, B., LeBoeuf, E. J., Pan, H., & Dai, S. (2002). Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere, 48(1), 59–68.
Collier, K. J., & Winterbourn, M. J. (1987). Faunal and chemical dynamics of some acid and alkaline New Zealand streams. Freshwater Biology, 18(2), 227–240.
Cooke, J. D., Hamilton-Taylor, J., & Tipping, E. (2007). On the acid–base properties of humic acid in soil. Environmental Science and Technology, 41, 465–470.
Davies, H., Weber, P., Lindsay, P., Craw, D., Peake, B., & Pope, J. (2011). Geochemical changes during neutralization of acid mine drainage in a dynamic mountain stream, New Zealand. Applied Geochemistry, 26(12), 2121–2133.
Dries, J., Bastiaens, L., Springael, D., Kuypers, S., Agathos, S. N., & Diels, L. (2005). Effect of humic acids on heavy metal removal by zero-valent iron in batch and continuous flow column systems. Wat. Res., 39, 3531–3540.
Garcia-Sanchez, A., & Álvarez-Ayuso, E. (2002). Sorption of Zn, Cd and Cr on calcite. Application to purification of industrial wastewaters, Minerals Engineering, 15(7), 539–547.
Greig, H. S., Niyogi, D. K., Hogsden, K. L., Jellyman, P. G., & Harding, J. S. (2010). Heavy metals: confounding factors in the response of New Zealand freshwater fish assemblages to natural and anthropogenic acidity. The Science of the Total Environment, 408(16), 3240–3250.
Gundersen, D., Bustaman, S., Seim, W., & Curtis, L. (1994). pH, hardness and humic acid influence aluminium toxicity to rainbow trout (Oncorhynchus mykiss) in weakly alkaline waters. Canadian Journal of Fisheries and Aquatic Sciences, 51.
Gustafsson, J. P. (2008). 2010, Visual MINTEQ, Version 3.0. Department of Land and Water Resources Engineering, Royal Institute of. Stockholm, Sweden: Technology.
Havelcová, M., Mizera, J., Sýkorová, I., & Pekař, M. (2009). Sorption of metal ions on lignite and the derived humic substances. Journal of Hazardous Materials, 161(1), 559–564.
Herrmann, R., & Baumgartner, I. (1992). Aluminium species distribution during mixing of acid coal and slate mine drainage with neutral stream waters. International Journal of Earth Sciences, 81(3), 759–767.
Hur, J., & Schlautman, M. A. (2003). Molecular weight fractionation of humic substances by adsorption onto minerals. J. Colloid Interf. Sci., 264, 313–321.
Johnson, D. B., & Hallberg, K. B. (2005). Acid mine drainage remediation options: a review. The Science of the Total Environment, 338, 3–14.
Jung, A. V., Chanudet, V., Ghanbaja, J., Lartiges, B. S., & Bersillon, J. L. (2005). Coagulation of humic substances and dissolved organic with a ferric salt: an electron energy loss spectroscopy investigation. Water Research, 39, 3849–3862.
Lee, G., Bigham, J. M., & Faure, G. (2002). Removal of trace metals by coprecipitation with Fe, Al and Mn from natural waters contaminated with acid mine drainage in the Ducktown Mining District, Tennessee. Applied Geochemistry, 17(5), 569–581.
Lee, Y. J., Elzinga, E. J., & Reeder, R. J. (2005). Cu(II) adsorption at the calcite–water interface in the presence of natural organic matter: kinetic studies and molecular-scale characterization. Geochimica et Cosmochimica Acta, 69(1), 49–61.
Linnik, P. N., & Vasilchuk, T. A. (2005). Role of humic substances in the complexation and detoxification of heavy metals: case study of the Dnieper Reservoirs. In I. V. Perminova, K. Hatfield, & N. Hertkorn (Eds.), Use of humic substances to remediate polluted environments: from theory to practice (p. 52). Dordrecht, The Netherlands: Springer.
Liu, T., Tsang, D. C. W., & Lo, I. M. C. (2008). Chromium(VI) reduction kinetics by zero-valent iron in moderately hard water with humic acid: humic acid adsorption and iron dissolution. Environmental Particulate Science and Technology, 42, 2092–2098.
Meador, J. P. (1991). The interaction of pH, dissolved organic carbon, and total copper in the determination of ionic copper and toxicity. Aquatic Toxicology, 19(1), 13–31.
Milne, C. J., Kinniburgh, D. G., & Tipping, E. (2001). Generic NICA–Donnan model parameters for proton binding by humic substances. Environmental Science and Technology, 35, 2049–2059.
Milne, C. J., Kinniburgh, D. G., Van Riemsdijk, W. H., & Tipping, E. (2003). Generic NICA–Donnan model parameters for metal–ion binding by humic substances. Environmental Science and Technology, 37, 958–971.
Monteil-Rivera, F., Brouwer, E. B., Masset, S., Deslandes, Y., & Dumonceau, J. (2000). Combination of X-ray photoelectron and solid-state 13C nuclear magnetic resonance spectroscopy in the structural characterisation of humic acids. Analytica Chimica Acta, 424(2), 243–255.
Pehlivan, E., & Arslan, G. (2007). Removal of metal ions using lignite in aqueous solution—Low cost biosorbents. Fuel Processing Technology, 88(1), 99–106.
Pope, J., Newman, N., Craw, D., Trumm, D., & Rait, R. (2010). Factors that influence coal mine drainage chemistry West Coast, South Island, New Zealand. New Zealand Journal of Geology and Geophysics, 53(2–3), 115–128.
Romkens, P. F. A. M., & Dolfing, J. (1998). Effect of Ca on the solubility and molecular size distribution of DOC and Cu binding in soil solution samples. Environmental Science and Technology, 32, 363–369.
Rouff, A. A., Elzinga, E. J., Reeder, R. J., & Fisher, N. S. (2005). The influence of pH on the kinetics, reversibility and mechanisms of Pb(II) sorption at the calcite-water interface. Geochimica et Cosmochimica Acta, 69(22), 5173–5186.
Ryan, A. C., Van Genderen, E. J., Tomasso, J. R., & Klaine, S. J. (2004). Influence of natural organic matter source on copper toxicity to larval fathead minnows (Pimephales promelas): implications for the biotic ligand model. Environmental Toxicology and Chemistry, 23(6), 1567–1574.
Salati, S., Adani, F., Cosentino, C., & Torri, G. (2008). Studying soil organic matter using 13C CP-MAS NMR: the effect of soil chemical pre-treatments on spectra quality and representativity. Chemosphere, 70(11), 2092–2098.
Schnitzer, M., & Kahn, S. U. (1972). Humic substances in the environment. New York: Dekker.
Schwartz, M. L., Curtis, P. J. , & Playle, R. C. (2004). Influence of natural organic matter source on acute copper, lead, and cadmium toxicity to rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 23(12), 2889–2899.
Stevenson, F. J. (1994). Humus Chemistry: Genesis, Composition, Reactions. John Wiley & Sons, Inc.
Tsang, D. C. W., Graham, N. J. D., & Lo, I. M. C. (2009). Humic acid aggregation in zero-valent iron systems and its effects on trichloroethylene removal. Chemosphere, 75, 1338–1343.
Tipping, E. (2002). Cation binding by humic substances. UK: Cambridge University Press Cambridge. 528pp.
Trumm, D. (2010). Selection of active and passive treatment systems for AMD—flow charts for New Zealand conditions. New Zealand Journal of Geology and Geophysics, 53(2–3), 195–210.
Warren, L. A., & Zimmerman, A. P. (1994). Suspended particulate oxides and organic matter interactions in trace metal sorption reactions in a small urban river. Biogeochemistry, 24(1), 21–34.
Weber, W. J., Tang, J., & Huang, Q. (2006). Development of engineered natural organic sorbents for environmental applications: 1. Materials, approaches, and characterizations. Environmental Science and Technology, 40(5), 1650–1656.
Webster, J. G., Swedlund, P. J., & Webster, K. S. (1998). Trace metal adsorption onto an acid mine drainage iron(III) oxy hydroxy sulfate. Environmental Science and Technology, 32(10), 1361–1368.
Winterbourn, M. J., McDiffett, W. F., & Eppley, S. J. (2000). Aluminium and iron burdens of aquatic biota in New Zealand streams contaminated by acid mine drainage: effects of trophic level. The Science of the Total Environment, 254(1), 45–54.
Yim, J. H., Kim, K. W., & Kim, S. D. (2006). Effect of hardness on acute toxicity of metal mixtures using Daphnia magna: prediction of acid mine drainage toxicity. Journal of Hazardous Materials, 138(1), 16–21.
Acknowledgements
The authors thank the Foundation for Research Science and Technology Fund provided by Royal Society of New Zealand for the financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 715 kb)
Technical Appendices
Technical Appendices
Supplementary technical materials are available online.
Rights and permissions
About this article
Cite this article
Olds, W.E., Tsang, D.C.W. & Weber, P. Acid Mine Drainage Treatment Assisted by Lignite-Derived Humic Substances. Water Air Soil Pollut 224, 1521 (2013). https://doi.org/10.1007/s11270-013-1521-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1521-5