Abstract
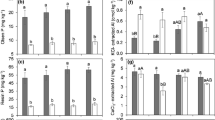
Lime was investigated as a soil amendment to decrease phosphorus (P) loss in runoff from two Delaware sandy loam soils, one high and one low in P. Soils were limed at three rates (control and target pH values of 6 and 6.8, respectively), packed into runoff boxes (2,000 cm2) and received simulated rainfall (80 mm h−1 for 30 min). Lime showed potential to decrease P loss in runoff, but its effectiveness was soil specific and dependant on other management factors also. Lime decreased dissolved reactive P (DRP) and dissolved organic P (DOP) loss by 20–25 and 52–93 %, respectively, for the high-P soil and particulate P (PP) by 13 % for the low-P soil. The majority of P lost in runoff was DOP (3–29 %) or PP (64–96 %). Lime increased PP losses from the finer-textured soil following P application, indicating that increased P sorption can lead to increased losses if P is sorbed to more erodable particles. Initial soil P status was more important than liming in determining P loss. While amendments may decrease P losses in the short term, addressing nutrient imbalances at the field scale is clearly necessary in the long term. Losses increased significantly following inorganic P application. Although P was sorbed rapidly, with less than 2 % of added P removed in runoff, mean concentrations in excess of 700 μg l−1 DRP, 2,500 μg l−1 OP and 6,500 μg l−1 PP were recorded for both soils immediately following P application.




Similar content being viewed by others
References
Avery, B. W., & Bascomb, C. L. (1974). Soil survey technical monograph no.6, soil survey laboratory methods. Dorking: Adlard & Son Ltd.
Boesch, D. F., Brinsfield, R. B., & Magnien, R. E. (2001). Chesapeake bay eutrophication: scientific understanding, ecosystem restoration, and challenges for agriculture. Journal of Environmental Quality, 30(2), 303–320.
Boruvka, L., & Rechcigal, J. E. (2003). Phosphorus retention by the Ap horizon of a spodosol as influenced by calcium amendments. Soil Science, 168(10), 699–706.
Callahan, M. P., Kleinman, P. J. A., Sharpley, A. N., & Stout, W. L. (2002). Assessing the efficacy of alternative phosphorus sorbing soil amendments. Soil Science, 167(8), 539–547.
Cox, J. W., Varcoe, J. C. R., Chittleborough, D. J., & van Leeuwen, J. (2005). Using gypsum to reduce phosphorus in runoff from subcatchments in South Australia. Journal of Environmental Quality, 34, 2118–2128.
Curtin, D., & Syers, J. K. (2001). Lime-induced changes in indices of soil phosphate availability. Soil Science Society of America Journal, 65, 147–152.
Eckert, D., & Sims, J. T. (1995). Recommended soil pH and lime requirement tests. Recommended soil testing procedures for the Northeastern United States. Northeastern Regional Publication.
Hannapel, R. J., Fuller, W. H., & Fox, R. H. (1964). Phosphorus movement in a calcareous soil: II. Soil microbial activity and organic phosphorus movement. Soil Science, 97, 421–427.
Haygarth, P. M., & Jarvis, S. C. (1999). Transfer of phosphorus from agricultural soils. Advances in Agronomy, 66, 195–249.
Johnson, K. N., Allen, A. L., Kleinman, P. J. A., Hashem, F. M., Sharpley, A. N., & Stout, W. L. (2011). Effect of coal combustion by-products on phosphorus runoff from a coastal plain soil. Communications in Soil Science and Plant Analysis, 42(7), 778–789.
Lopez-Pineiro, A., Cabrera, D., Pena, D., Albarran, A., & Rato Nunes, J. M. (2009). Phosphorus adsorption and fractionation in a two-phase olive mill waste amended soil. Soil Science Society of America Journal, 73(5), 1539–1544. doi:10.2136/sssaj2009.0035.
MAFF. (1986). The analysis of agricultural materials. London: HMSO.
Maguire, R. O., Foy, R. H., Bailey, J. S., & Sims, J. T. (2001). Estimation of the phosphorus sorption capacity of acidic soils in Ireland. European Journal of Soil Science, 52, 479–487.
Maguire, R. O., Sims, J. T., & Foy, R. H. (2001). Long-term kinetics for phosphorus sorption–desorption by high phosphorus soils from Ireland and the Delmarva peninsula, USA. Soil Science, 166(8), 557–565.
McDowell, R. W. (2003). Identification of phosphorus species in extracts of soils with contrasting management histories. Communications in Soil Science and Plant Analysis, 34(7 and 8), 1083–1095.
McDowell, R., Sharpley, A., Brookers, P., & Poulton, P. (2001). Relationship between soil test phosphorus and phosphorus release to solution. Soil Science, 166(2), 137–149.
McKeague, J. A., & Day, J. H. (1966). Dithionite- and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Canadian Journal of Soil Science, 46, 13–22.
Mehlich, A. (1984). Mehlich 3 soil test extractant: a modification of the Mehlich 2 extractant. Communications in Soil Science and Plant Analysis, 15, 1409–1416.
Murphy, P. N. C. (2007). Lime and cow slurry application temporarily increases organic phosphorus mobility in an acidic soil. European Journal of Soil Science, 58, 794–801.
Murphy, J., & Riley, J. P. (1962). A modified single solution for the determination of phosphorus in natural waters. Analytica Chemica Acta, 27, 31–36.
Murphy, P. N. C., & Stevens, R. J. (2010). Lime and gypsum as source measures to decrease phosphorus loss from soils to water. Water, Air, and Soil Pollution, 212(1–4), 101–111. doi:10.1007/s11270-010-0325-0.
Murphy, P. N. C., Bell, A., & Turner, B. L. (2009). Phosphorus speciation in temperate basaltic grassland soils by solution 31P NMR spectroscopy. European Journal of Soil Science, 60, 638–651. doi:10.1111/j.1365-2389.2009.01148.x.
Muukkonen, P., Hartikainen, H., & Alakukku, L. (2009). Effect of soil structure disturbance on erosion and phosphorus losses from Finnish clay soil. Soil and Tillage Research, 103(1), 84–91.
Olsen, S. R., & Sommers, L. E. (1982). Phosphorus. In A. L. Page, R. H. Miller, & D. R. Keeney (Eds.), Methods of soil analysis (Agronomy Series 2nd ed., Vol. 9, pp. 403–430). Madison: Soil Science Society of America.
Olsen, S. R., Cole, C. V., Watanabe, E. S., & Deane, L. A. (1957). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture Circular.
Ortas, I., & Rowell, D. L. (2000). Effect of pH on amount of phosphorus extracted by 10 mM calcium chloride from three Rothamsted soils. Communications in Soil Science and Plant Analysis, 31, 2917–2923.
Penn, C. J., & Sims, J. T. (2002). Phosphorus forms in biosolids-amended soils and losses in runoff: effects of wastewater treatment process. Journal of Environmental Quality, 31(4), 1349–1361.
Peters, J. M., & Basta, N. T. (1996). Reduction of excess bioavailable phosphorus in soils by using municipal and industrial wastes. Journal of Environmental Quality, 25, 1236–1241.
Self-Davis, M. L., Moore, P. A., & Joern, B. C. (2000). Determination of water- and/or dilute salt-extractable phosphorus. In G. M. Pierzynski (Ed.), Methods of phosphorus analysis for soils, sediments, residuals, and waters (Southern Cooperative Series, Vol. 396). North Carolina: North Carolina State University.
Shand, C. A., & Smith, S. (1997). Enzymatic release of phosphate from model substrates and P compounds in soil solution from a peaty podzol. Biology and Fertility of Soils, 24, 183–187.
Sharpley, A. N., & Rekolainen, S. (1997). Phosphorus in agriculture and its environmental implications. In H. Tunney, O. T. Carton, P. C. Brookes, & A. E. Johnston (Eds.), Phosphorus loss from soil to water (pp. 1–53). New York: CAB International.
Sharpley, A., & Tunney, H. (2000). Phosphorus research strategies to meet agricultural and environmental challenges of the 21st century. Journal of Environmental Quality, 29, 176–181.
Sims, J. T. (2000). Soil test P: Mehlich 3. In G. M. Pierzynski (Ed.), Methods of phosphorus analysis for soils, sediments, residuals and waters (Southern Cooperative Series, Vol. 396, pp. 17–19). North Carolina: North Carolina State University.
Sims, J. T., Maguire, R. O., Leytem, A. B., Gartley, K. L., & Pautler, M. C. (2002). Evaluation of Mehlich 3 as an agri-environmental soil phosphorus test for the mid-atlantic United States of America. Soil Science Society of America Journal, 66(6), 2016–2032. doi:10.2136/sssaj2002.2016.
Toor, G. S., Condron, L. M., Di, H. J., Cameron, K. C., & Cade-Menum, B. J. (2003). Characterisation of organic phosphorus in leachate from a grassland soil. Soil Biology and Biochemistry, 35, 1317–1323.
Torbert, H. A., King, K. W., & Harmel, R. D. (2005). Impact of soil amendments on reducing phosphorus losses from runoff in sod. Journal of Environmental Quality, 34, 1415–1421.
Turner, B. L., Mahieu, N., & Condron, L. M. (2003). Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH-EDTA extracts. Soil Science Society of America Journal, 67, 497–510.
Tyler, G., & Olsson, T. (2001). Concentrations of 60 elements in the soil solution as related to the soil acidity. European Journal of Soil Science, 52, 151–165.
Varcoe, J. C. R., Chittleborough, D. J., Cox, J. W., & van Leeuwen, J. (2001). The effect of Ca soil amendments on P mobility. In P. M. Haygarth, L. M. Condron, P. J. Butler, & J. S. Chisholm (Eds.), International Phosphorus Transport Workshops 2001 (p. 89). Plymouth: Robbins Centre, Plymouth University.
White, J. W., Coale, F. J., Sims, J. T., & Shober, A. L. (2010). Phosphorus runoff from waste water treatment biosolids and poultry litter applied to agricultural soils. Journal of Environmental Quality, 39(1), 314–323. doi:10.2134/jeq2009.0106.
Acknowledgements
The authors wish to acknowledge the contribution of Dr. Jim Stevens (Queens University Belfast, retired) to this work and the help of Michelle Allen (Q.U.B.) with statistics, Dr. Ben Turner (Smithsonian Tropical Research Institute) with NMR analysis and the laboratory staff at Q.U.B. and U.D. with sample analysis. This project was funded by a U.S.D.A. grant at Q.U.B. and U.D. as part of a Ph.D. thesis funded by the Thomas Henry Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murphy, P.N.C., Sims, J.T. Effects of Lime and Phosphorus Application on Phosphorus Runoff Risk. Water Air Soil Pollut 223, 5459–5471 (2012). https://doi.org/10.1007/s11270-012-1293-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-012-1293-3