Abstract
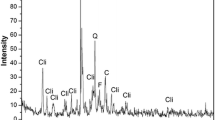
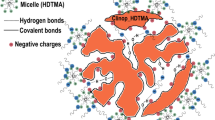
Natural clinoptilolite was modified with hexadecyltrimethylammonium chloride, a cationic surfactant, and then melt-mixed with polypropylene hollow fibres to produce polymer composites with adsorptive properties. The performance of the fabricated composites was evaluated by optimizing experimental parameters such as surfactant loading, contact time, pH and initial concentration for the adsorptive removal of 2,4,6-trichlorophenol (TCP) and ortho-nitrophenol (o-NP). Based on the fourier transmission infrared spectra and scanning electron microscopy micrographs of as-received and surfactant-modified clinoptilolite, the modification of natural clinoptilolite was attained. The composites showed enhanced adsorption capability for TCP over o-NP with removal efficiencies of 84% and 46%. Loading the clinoptilolite with surfactant concentrations beyond 8 mM reduced the adsorption capacity. The removal of TCP and o-NP was found to depend critically on the pH of the solution, and the optimum ranges were 4–6 and 2–6 for compounds, respectively. The adsorption dynamics were determined with first- and second-order kinetics models, and the adsorption system for TCP and o-NP followed the first-order kinetics. Adsorption isotherm analysis revealed that the adsorption equilibrium data obeyed/fit the Freundlich isotherm.













Similar content being viewed by others
Abbreviations
- AR:
-
As-received
- CLI:
-
Clinoptilolite
- CLI–PP:
-
Clinoptilolite–polypropylene composite
- CMC:
-
Critical micelle concentration
- ECEC:
-
External cationic exchange capacity
- HDTMA:
-
Hexadecyltrimethylammonium
- o-NP:
-
ortho-nitrophenol
- PP:
-
Polypropylene
- PPHF:
-
Polypropylene hollow fibre
- SEM:
-
Scanning electron microscopy
- SM CLI:
-
Surfactant-modified clinoptilolite
- SM CLI–PP:
-
Surfactant-modified clinoptilolite–polypropylene
- SM CLI–PPHF:
-
Surfactant-modified clinoptilolite–polypropylene hollow fibres
- TCP:
-
2,4,6-Trichlorophenol
- XRD:
-
X-ray diffraction
- XRF:
-
X-ray fluorescence
- FTIR:
-
Fourier transform infrared spectroscopy
- UV–vis:
-
Ultraviolent–visible spectrophotometer
- rpm:
-
Revolutions per minute
References
Ahmaruzzaman, M. (2008). Adsorption of phenolic compounds on low-cost adsorbents: a review. Advances in Colloid and Interface Science, 143, 48–67.
Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials, 97, 219–243.
Bowman, R. S. (2000). Sorption of ionizable organic solutes by surfactant-modified zeolite. Environmental Science & Technology, 34, 3756–3760.
Cakicioglu-Ozkan, F., & Ulku, S. (2005). The effect of HCl treatment on water vapor adsorption characteristics of clinoptilolite rich natural zeolite. Langmuir, 77, 47–53.
Chen, H., Zhou, W., Zhu, K., Zhan, H., & Jiang, M. (2004). Sorption of ionizable organic compounds on HDTMA-modified loess soil. Environment, 326, 217–223.
Christidis, G. E., Moraetis, D., Keheyan, E., & Akhalbedashvili, L. (2003). Chemical and thermal modification of natural HEU-type zeolitic materials from Armenia, Georgia and Greece. Applied Clay Science, 24, 79–91.
Chutia, P., Kato, S., Kojima, T., & Satokawa, S. (2009). Adsorption of As (V) on surfactant-modified natural zeolites. Journal of Hazardous Materials, 162, 204–211.
Crini, G. (2006). Non-conventional low-cost adsorbents for dye removal: a review. Bioresource Technology, 97, 1061–1085.
Dong, Y., Wu, D., Chen, X., & Lin, Y. (2010). Adsorption of bisphenol A from water by surfactant-modified zeolite. Journal of Colloidal & Interface Science, 348, 585–590.
Gacitua, W., Ballerini, A., & Zhang, J. (2005). Polymer nanocomposites: synthetic and natural fillers. Ciencia y Tecnología, 7(3), 159–178.
Godelitsas, A. (2003). HEU-type zeolites modified by transition elements and lead. Microporous and Mesoporous Materials, 61, 3–24.
Gupta, V. K. (2009). Application of low-cost adsorbents for dye removal—a review. Journal of Environmental Management, 90, 2313–2342.
Inglezakis, V. J., Loizidou, M. M., & Grigoropoulou, H. P. (2004). Ion exchange studies on natural and modified zeolites and the concept of exchange site accessibility. Journal of Colloidal & Interface Science, 275(2), 570–576.
Jin, X., Jiang, M.-Q., Shan, X.-Q., Pei, Z.-G., & Chen, Z. (2008). Adsorption of methylene blue and orange II onto unmodified and surfactant-modified zeolite. Journal of Colloidal & Interface Science, 328, 243–247.
Kuleyin, A. (2007). Removal of phenol and 4-chlorophenol by surfactant-modified natural zeolite. Journal of Hazardous Materials, 144, 307–315.
Lagergren, S. (1898). Zur theorie der sogenannten adsorption gelöster stoffe, kungliga Swenska vetenskapsakademiens. Handingar, 24, 1–39.
Lemic, J., Tomaševic-Canovic, M., Djuricic, M., & Stanic, T. (2005). Surface modification of sepiolite with quaternary amines. Journal of Colloidal & Interface Science, 292, 11–19.
Lemic, J., Kovacevic, D., Tomasevic-Canovic, M., Kovacevic, D., Stanic, T., & Pfend, R. (2006). Removal of atrazine, lindane and diazinone from water by organo-zeolite. Water Research, 40, 1079–1085.
Li, Z. (1999). Sorption kinetics of hexadecyltrimethylammonium on natural clinoptilolite. Langmuir, 15(19), 6438–6445.
Li, Z., & Bowman, R. S. (1998). Sorption of perchloroethylene by surfactant-modified zeolite as controlled by surfactant loading, Environmental Science & Technology, 32, 2278–2282.
Penalver, A., Pocurull, E., Borrull, F., & Marce, R. M. (2002). Solid-phase microextraction coupled to high-performance liquid chromatography to determine phenolic compounds in water samples. Journal of Chromatography A, 953, 79–87.
Radhika, M., & Palanivelu, K. (2006). Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent—kinetics and isotherm analysis. Journal of Hazardous Materials, 138, 116–124.
Safe, S. (1993). Toxicology, structure–function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environmental Health, 100, 259–268.
Shu, H.-T., Li, D., Scala, A. A., & Hua, Y. (1997). Adsorption of small organic pollutants from aqueous streams by aluminosilicate-based microporous materials. Separation and Purification Technology, 11, 27–36.
Sprynskyy, M., Ligor, T., & Buszewski, B. (2008). Clinoptilolite in the study of lindane and aldrin sorption processes from water solution. Journal of Hazardous Materials, 151, 570–577.
Sprynskyy, M., Ligor, T., Lebedynets, M., & Buszewski, B. (2009). Kinetic and equilibrium studies of phenol adsorption by natural and modified forms of the clinoptilolite. Journal of Harzadous Materials, 169, 847–854.
Tang, X., Zhou, Y., Xu, Y., Zhao, Q., Zhou, X., & Lu, J. (2010). Sorption of polycyclic aromatic hydrocarbons from aqueous solution by hexadecyltrimethylammonium bromide modified fibric peat. Journal of Chemical Technology & Biotechonogy, 85(8), 1084–1091.
Tomasevic-canovic, M. R., & Dondur, V. T. (2000). The adsorption of sulphate, hydrogenchromate and dihydrogenphosphate anions on surfactant-modified clinoptilolite. Applied Clay Science, 2000, 265–277.
Trgo, M., Peri, J., & Vukojevi, N. (2006). A comparative study of ion exchange kinetics in zinc/lead—modified zeolite-clinoptilolite systems. Journal of Hazardous Materials, 136, 938–945.
Tsai, W.-T., Hsien, K.-J., & Hsu, H-Ch. (2009). Adsorption of organic compounds from aqueous solution onto the synthesized zeolite. Langmuir, 166, 635–641.
Ulusoy, U. (2005). Lead removal by polyacrylamide-bentonite and zeolite composites: effect of phytic acid immobilization. Langmuir, 127, 163–171.
Vujakovic, A. A., Dakovic, A., Lemic, J., Radosaljevic-Mihajlovic, A., & Tomacevi-Canovic, M. (2003). Adsorption of inorganic anionic contaminants on surfactant modified minerals. Journal of Serbian Chemical Society, 68, 833–841.
Wang, S., & Peng, Y. (2010). Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engeneering Journal, 156, 11–24.
Wingenfelder, U., & Schulin, R. (2005). Removal of heavy metals from mine waters by natural zeolites. Environmental Science & Technology, 39, 4606–4613.
Wingenfelder, U., Furrer, G., & Schulin, R. (2006). Sorption of antimonate by HDTMA-modified zeolite. Microporous and Mesoporous Materials, 95, 265–271.
Yousef, R. I., & El-Eswed, B. (2009). The effect of pH on the adsorption of phenol and chlorophenols onto natural zeolite. Colloids and Surfaces A: Physicochemical & Engeneering Aspects, 334, 92–99.
Zou, Y., & Bowman, R. S. (1998). Long-term chemical and biological stability of surfactant-modified zeolite. Environmental Science & Technology, 32, 2628–2632.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Motsa, M.M., Thwala, J.M., Msagati, T.A.M. et al. Adsorption of 2,4,6-Trichlorophenol and ortho-Nitrophenol from Aqueous Media Using Surfactant-Modified Clinoptilolite–Polypropylene Hollow Fibre Composites. Water Air Soil Pollut 223, 1555–1569 (2012). https://doi.org/10.1007/s11270-011-0964-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0964-9