Abstract
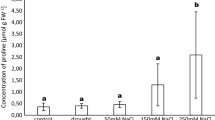
There is increasing interest in poplars and willows due to their biomass production and phytoremediation potential. They host two major types of mycorrhizal fungi that can substantially modulate the physiology of their hosts. In this study, the effects of endo- and ectomycorrhizal fungi on growth, physiological parameters, and heavy metals accumulation were studied in a pot experiment using Salix alba L. and Populus nigra L. The mycorrhizal fungi were inoculated separately and in combination to a soil substrate polluted by a mixture of heavy metals (mainly Cd, Pb, and Zn). Tree species differed in their mycorrhizal affinities, with poplar being colonized predominantly by Glomus intraradices and willow by Hebeloma mesophaeum. H. mesophaeum increased willow height and biomass, while G. intraradices decreased poplar height. The photosynthetic rate remained unchanged, and only minor changes were observed in the relative composition of photosynthetic pigments. Poplar photosynthetic rates and levels of photosynthetic pigments declined, while the epicuticular waxes in leaves increased toward the end of the experiment, irrespective of the inoculation. H. mesophaeum strongly reduced the accumulation of Cd and Fe in willow and poplar shoots, respectively. Our results support the use of selected mycorrhizal strains to tune phytoremediation outcomes in their plant hosts.


Similar content being viewed by others
References
Adriaensen, K., Vangronsveld, J., & Colpaert, J. V. (2006). Zinc-tolerant Suillus bovinus improves growth of Zn-exposed Pinus sylvestris seedlings. Mycorrhiza, 16, 553–558.
Aguillon, R. L., & Garbaye, J. (1989). Some aspects of a double symbiosis with ectomycorrhizal and VAM fungi. Agriculture, Ecosystem and Environment, 29, 263–266.
Arriagada, C. A., Herrera, M. A., & Ocampo, J. A. (2005). Contribution of arbuscular mycorrhizal and saprobe fungi to the tolerance of Eucalyptus globulus to Pb. Water, Air, and Soil Pollution, 166, 31–47.
Audet, P., & Charest, Ch. (2007). Heavy metal phytoremediation from a meta-analytical perspective. Environmental Pollution, 147, 231–237.
Baum, Ch, & Makeschin, F. (2000). Effects of nitrogen and phosphorus fertilization on mycorrhizal formation of two poplar clones (Populus trichocarpa and P. tremula x tremuloides). Journal of Plant and Nutrition Soil Science, 163, 491–497.
Baum, Ch, Hrynkiewicz, K., Leinweber, P., & Meißner, R. (2006). Heavy-metal mobilization and uptake by mycorrhizal and nonmycorrhizal willows (Salix × dasyclados). Journal of Plant and Nutrition Soil Science, 169, 516–522.
Bellion, M., Courbot, M., Jacob, Ch, Blaudez, D., & Chalot, M. (2006). Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiology Letters, 254, 173–181.
Bissonnette, L., St-Arnaud, M., & Labrecque, M. (2010). Phytoextraction of heavy metals by two Salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial. Plant and Soil, 332, 55–67.
Castiglione, S., Franchin, C., Fossati, T., Lingua, G., Torrigiani, P., & Biondi, P. (2007). High zinc concentrations reduce rooting capacity and alter metallothionein gene expression in white poplar (Populus alba L. cv. Villafranca). Chemosphere, 67, 1117–1126.
Castiglione, S., Todeschini, V., Franchin, C., Torrigiani, P., Gastaldi, D., Cicatelli, A., et al. (2009). Clonal differences in survival capacity, copper and zinc accumulation, and correlation with leaf polyamine levels in poplar: A large-scale field trial on heavily polluted soil. Environmental Pollution, 157, 2108–2117.
Chilvers, G. A., Lapeyrie, F. F., & Horan, D. P. (1987). Ectomycorrhizal vs endomycorrhizal fungi within the same root system. The New Phytologist, 107, 441–448.
Cicatelli, A., Lingua, G., Todeschini, V., Biondi, S., Torrigiani, P., & Castiglione, S. (2010). Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Annals of Botany. doi:10.1093/aob/mcq170.
Di Baccio, D., Tognetti, R., Minnocci, A., & Sebastiani, L. (2009). Responses of the Populus × euramericana clone I-214 to excess zinc: Carbon assimilation, structural modifications, metal distribution and cellular localization. Environmental and Experimental Botany, 67, 153–163.
Dickinson, N. M. (2006). Phytoremediation of industrially-contaminated sites using trees. NATO Science Series, 68, 229–240.
Dos Santos Utmazian, M. N., Schweiger, P., Sommer, P. M., Gorfer, M., Strauss, J., & Wenzel, W. W. (2007). Influence of Cadophora finlandica and other microbial treatments on cadmium and zinc uptake in willows grown on polluted soil. Plant and Soil Environment, 53, 158–166.
French, Ch. J., Dickinson, N. M., & Putwain, P. D. (2006). Woody biomass phytoremediation of contaminated brownfield land. Environmental Pollution, 141, 387–395.
Gehring, C. A., Mueller, C., & Whitham, T. G. (2006). Environmental and genetic effects on the formation of ectomycorrhizal and arbuscular mycorrhizal associations in cottonwoods. Oecologia, 149, 158–164.
Giovannetti, M., & Mosse, B. (1980). An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. The New Phytologist, 84, 489–500.
Harley, J. L., & Harley, E. L. (1987). A check-list of mycorrhiza in the British Flora. The New Phytologist, 105(Suppl), 1–120.
Hashimoto, Y., & Higuchi, R. (2003). Ectomycorrhizal and arbuscular mycorrhizal colonization of two species of floodplain willows. Mycoscience, 44, 339–343.
Hrynkiewicz, K., Haug, I., & Baum, Ch. (2008). Ectomycorrhizal community structure under willows at former ore mining sites. European Journal of Soil Biology, 44, 37–44.
Jentschke, G., & Godbold, D. L. (2000). Metal toxicity and ectomycorrhizas. Physiologia Plantarum, 109, 107–116.
Keller, C., Ludwig, Ch, Davoli, F., & Wochele, J. (2005). Thermal treatment of metal-enriched biomass produced from heavy metal phytoextraction. Environmental Science and Technology, 39, 3359–3367.
Khasa, P. D., Chakravarty, P., Robertson, A., Thomas, B. R., & Dancik, B. P. (2002). The mycorrhizal status of selected poplar clones introduced in Alberta. Biomass and Bioenergy, 22, 99–104.
Koske, R. E., & Gemma, J. N. (1989). A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research, 92, 486–488.
Krpata, D., Peinter, U., Langer, I., Fitz, W. J., & Schweiger, P. (2008). Ectomycorrhizal communities associated with Populus tremula growing on a heavy metal contaminated site. Mycological Research, 112, 1069–1079.
Leyval, C., Turnau, K., & Haselwandter, K. (1997). Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza, 7, 139–153.
Li, H., Smith, F. A., Dickson, S., Holloway, R. E., & Smith, S. E. (2008). Plant growth depressions in arbuscular mycorrhizal symbioses: not just caused by carbon drain? The New Phytologist, 178, 852–862.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382.
Lingua, G., Franchin, C., Todeschini, V., Castiglione, S., Biondi, S., Burlando, B., et al. (2008). Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environmental Pollution, 153, 137–147.
Lodge, D. J. (1989). The influence of soil moisture and flooding on formation of VA-endo- and ectomycorrhizae in Populus and Salix. Plant and Soil, 117, 243–253.
Lodge, D. J., & Wentworth, T. R. (1990). Negative associations among VA-mycorrhizal fungi and some ectomycorrhizal fungi inhabiting the same root system. Oikos, 57, 347–356.
Lukac, M., Calfapietra, C., & Godbold, D. L. (2003). Production, turnover and mycorrhizal colonization of root systems of three Populus species grown under elevated CO2 (POPFACE). Global Change Biology, 9, 838–848.
Lunáčková, L., Masarovičová, E., Kráľová, K., & Streško, V. (2003). Response of fast growing woody plants from family Salicaceae to cadmium treatment. Bulletin of Environmental Contamination and Toxicology, 70, 576–585.
Matějka, P., Plešerová, L., Budínová, G., Havířová, K., Mulet, X., Skácel, F., et al. (2001). Vibrational biospectroscopy: what can we say about the surface wax layer of Norway spruce needles? Journal of Molecular Structure, 565–566, 305–310.
Negri, M. C., Gatliff, E. G., Quinn, J. J., & Hinchman, R. R. (2003). Root development and rooting at depths. In S. C. McCutcheon & J. L. Schnoor (Eds.), Phytoremediation transformation and control of contaminants (pp. 233–262). Hoboken: Wiley.
Neville, J., Tessier, J. L., Morrison, I., Scarratt, J., Canning, B., & Klironomos, J. N. (2002). Soil depth distribution of ecto- and arbuscular mycorrhizal fungi associated with Populus tremuloides within a 3-year-old boreal forest clear-cut. Applied Soil Ecology, 19, 209–216.
Parádi, I., & Baar, J. (2006). Mycorrhizal fungal diversity in willow forests of different age along the river Waal, The Netherlands. Forest Ecology and Management, 237, 366–372.
Pulford, I. D., & Watson, C. (2003). Phytoremediation of heavy metal-contaminated land by trees-review. Environment International, 29, 529–540.
Regvar M., Likar M., Piltaver A., Kugonič N., & Smith J. E. (2010) Fungal community structure under goat willows (Salix caprea L.) growing at metal polluted site: the potential of screening in a model of phytostabilisation study. Plant and Soil, 330, 345–356.
Schnitzler, A. (1997). River dynamics as a forest process: interaction between fluvial systems and alluvial forests in large European river plains. The Botanical Review, 63, 40–64.
Schützendübel, A., & Polle, A. (2002). Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany, 53, 1351–1365.
Sell, J., Kayser, A., Schulin, R., & Brunner, I. (2005). Contribution of ectomycorrhizal fungi to cadmium uptake of poplars and willows from a heavily polluted soil. Plant and Soil, 277, 245–253.
Smith, S. E., & Read, D. J. (1997). Mycorrhizal symbiosis (2nd ed.). London, UK: Academic Press Ltd.
Sudová, R., Doubková, P., & Vosátka, M. (2008). Mycorrhizal association of Agrostis capillaris and Glomus intraradices under heavy metal stress: combination of plant clones and fungal isolates from contaminated and uncontaminated substrates. Applied Soil Ecology, 40, 19–29.
Tlustoš, P., Száková, J., Vysloužilová, M., Pavlíková, D., Weger, J., & Javorská, H. (2007). Variation in the uptake of arsenic, cadmium, lead, and zinc by different species of willows Salix spp. grown in contaminated soils. Central European Journal of Biology, 2, 254–275.
van der Heijden, E. W. (2001). Differential benefits of arbuscular mycorrhizal and ectomycorrhizal infection of Salix repens. Mycorrhiza, 10, 185–193.
van der Heijden, E. W., & Vosátka, M. (1999). Mycorrhizal associations of Salix repens L. communities in succession of dune ecosystems. II. Mycorrhizal dynamics and interactions of ectomycorrhizal and arbuscular mycorrhizal fungi. Canadian Journal of Botany, 77, 1833–1841.
Vozzo, J. A., & Hacskaylo, E. (1974). Endo- and ectomycorrhizal associations in five Populus species. Bulletin of the Torrey Botanical Club, 101, 182–186.
Zimmer, D., Baum, Ch, Leinweber, P., Hrynkiewicz, K., & Meissner, R. (2009). Associated bacteria increase the phytoextraction of cadmium and zinc from metal-contaminated soil by mycorrhizal willows. International Journal of Phytoremediation, 11, 200–213.
Acknowledgments
We greatly acknowledge the support of the research by a grant from Norway through the Norwegian Financial Mechanism (project no. CZ0092), by the Ministry of Education, Youth and Sports of the Czech Republic (MSM 6046137307), and by the Academy of Sciences of the Czech Republic (grant AV0Z60050516). We also thank Dr. Radka Sudová for the selection of the AM strains, Mgr. Pavla Doubková for the preparation of the AM inocula, and Dr. Andrea Polle for kindly providing us the isolate P. involutus Maj.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
The x-loadings for principal component 1 (PC1) and 2 (PC2) of FT-Raman spectra (JPEG 62 kb)
Rights and permissions
About this article
Cite this article
Mrnka, L., Kuchár, M., Cieslarová, Z. et al. Effects of Endo- and Ectomycorrhizal Fungi on Physiological Parameters and Heavy Metals Accumulation of Two Species from the Family Salicaceae. Water Air Soil Pollut 223, 399–410 (2012). https://doi.org/10.1007/s11270-011-0868-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0868-8