Abstract
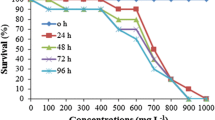
Inorganic fluoride concentrations in aquatic ecosystems have been significantly increased by several human activities during the last decades. However, there is still relatively scarce information about its toxicity to freshwater animals, especially at long-term exposures. The aim of our study is to assess the short-term (4 days) and long-term (28 days) fluoride (F−) toxicity to the aquatic snail Potamopyrgus antipodarum on the basis of several endpoints, including survival, reproduction (number of newborn and embryos) and behaviour (time to start movement). One control and five actual fluoride concentrations were used in triplicate (49.2, 47.0, 122.5, 194.6 and 281.4 mg F−/L) for the short-term (4 days) bioassay. LC50 value at 96 h was 58.5 mg F−/L, which is a relatively high value in comparison with previous published data on freshwater invertebrates. One control and three actual fluoride concentrations were used in quadruplicate (4.6, 9.5 and 16.2 mg F−/L) for the long-term (28 days) bioassay. None of the fluoride treatments increased mortality in comparison to control after 28 days of continuous exposure. Fluoride reduced the mean total and alive number of newborns per surviving adult after 28 days of exposure (at mean concentrations of 9.5 and 16.2 mg F−/L) respect to control. The number of embryos with shell was reduced by the highest concentration (16.2 mg F−/L). The behavioural activity (e.g. time to start normal movement) was affected by the highest fluoride concentration during the long-term bioassay. Our results show that fluoride is toxic at short- and long-term exposures, causing mortality (at short term) and affecting reproduction and behaviour (at long term). Additionally, field fluoride levels, corresponding to test fluoride concentrations (mean values ranged from 4.6 to 16.2 mg F−/L) have been found in fluoride-polluted ecosystems, either by natural or anthropogenic causes. Therefore, fluoride pollution may potentially affect natural population of invertebrates.



Similar content being viewed by others
References
Adhikari, S., Naqvi, A. A., & Sarangi, N. (2006). Effect of fluoride on growth and feed intake of juvenile giant freshwater prawn Macrobrachium rosenbergii (De-Man). Fluoride, 39, 313–317.
Alonso, A. (2005). Valoración de la degradación ambiental y efectos ecotoxicológicos sobre la comunidad de macroinvertebrados bentónicos en la cabecera del río Henares. Ph.D. dissertation, Universiy of Alcalá, Spain, 197 pp.
Alonso, A., & Camargo, J. A. (2003). Short-term toxicity of ammonia, nitrite and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bulletin of Environmental Contamination and Toxicology, 70, 1006–1012.
Alonso, A., & Camargo, J. A. (2004). Sub-lethal responses of the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca) to unionized ammonia: A tolerant invading species. Fresenius’ Environmental Bulletin, 13, 607–615.
Alonso, A., & Camargo, J. A. (2009). Long-term effects of ammonia on the behavioral activity of the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Archives of Environmental Contamination and Toxicology, 56, 796–802.
Alonso, A., & Castro-Diez, P. (2008). What explains the invading success of the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia, 614, 107–116.
APHA, American Public Health Association. (1995). Standard methods for the examination of water and wastewater (19th ed.). Washington, DC: American Public Association.
Bhatnagar, C., Bhatnagar, M., & Regar, B. C. (2007). Fluoride-induced histopathological changes in gill, kidney, and intestine of fresh water teleost, Labeo rohita. Fluoride, 40, 51–61.
Camargo, J. A. (1996). Comparing levels of pollutants in regulated rivers with safe concentrations of pollutants for fishes: A case study. Chemosphere, 33, 81–90.
Camargo, J. A. (2003). Fluoride toxicity to aquatic organisms: A review. Chemosphere, 50, 251–264.
Camargo, J. A. (2004). Effects of body size and sodium chloride on the tolerance of net-spinning caddisfly larvae to fluoride toxicity. Bulletin of Environmental Contamination and Toxicology, 72, 579–585.
Camargo, J. A., & Tarazona, J. V. (1990). Acute toxicity to freshwater macroinvertebrates of fluoride ion (F− ) in soft water. Bulletin of Environmental Contamination and Toxicology, 45, 883–887.
Camargo, J. A., Ward, J. V., & Martin, K. L. (1992). The relative sensitivity of competing hydropsychid species to fluoride toxicity in the Cache la Poudre River (Colorado). Archives of Environmental Contamination and Toxicology, 22, 107–113.
Damkaer, D. M., & Dey, D. B. (1989). Evidence for fluoride effects on salmon passage at John Day Dam, Columbia River, 1982–1986. North American Journal of Fisheries Management, 9, 154–162.
Dave, G. (1984). Effects of fluoride on growth, reproduction and survival in Daphnia magna. Comparative Biochemistry and Physiology, 78C, 425–431.
Duft, M., Schulte-Oehlmann, U., Weltje, L., Tillmann, M., & Oehlmann, J. (2003). Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail Potamopyrgus antipodarum. Aquatic Toxicology, 64, 437–449.
Duft, M., Schmitt, C., Bachmann, J., Brandelik, C., Schulte-Oehlmann, U., & Oehlmann, J. (2007). Prosobranch snails as test organisms for the assessment of endocrine active chemicals—an overview and a guideline proposal for a reproduction test with the freshwater mudsnail Potamopyrgus antipodarum. Ecotoxicology, 16, 169–182.
Dybdahl, M. F., & Kane, S. L. (2005). Adaptation vs. phenotypic plasticity in the success of a clonal invader. Ecology, 86, 1592–1601.
Environment Canada. (2001). Canadian water quality guideline for the protection of aquatic life: Inorganic fluorides. Scientific Supporting Document. Ecosystem Health: Science-based Solutions Reports No 1-1. Ottawa: National Guideline and Standards Office, Environmental Quality Branch, Environment Canada.
Fieser, A. H., Sykora, J. L., Kostalos, M. S., Wu, Y. C., & Weyel, D. W. (1986). Effect of fluorides on survival and reproduction of Daphnia magna. Journal Water Pollution Control Federation, 58, 82–86.
Gust, M., Buronfosse, T., Giamberini, L., Ramil, M., Mons, R., & Garric, J. (2009). Effects of fluoxetine on the reproduction of two prosobranch mollusks: Potamopyrgus antipodarum and Valvata piscinalis. Environmental Pollution, 157, 423–429.
Herbst, D. B., Bogan, M. T., & Lusardi, R. A. (2008). Low specific conductivity limits growth and survival of the New Zealand mud snail from the upper Owens River, California. Western North American Naturalist, 68, 324–333.
Kühn, R., Patfard, M., Pernak, K.-D., & Winter, A. (1989). Results of the harmful effects of water pollutants to Daphnia magna in the 21 day reproduction test. Water Research, 23, 501–510.
LeBlanc, G. A. (1980). Acute toxicity of priority pollutants to water flea (Daphnia magna). Bulletin of Environmental Contamination and Toxicology, 24, 684–691.
Mazurová, E., Hilscherová, K., Jálová, V., Köhler, H. R., Triebskorn, R., Giesy, J. P., et al. (2008). Endocrine effects of contaminated sediments on the freshwater snail Potamopyrgus antipodarum in vivo in the cell bioassays in vitro. Aquatic Toxicology, 89, 172–179.
Metcalfe-Smith, J. L., Holtze, K. E., Sirota, G. R., Reid, J. J., & De Solla, S. R. (2003). Toxicity of aqueous and sediment-associated fluoride to freshwater organisms. Environmental Toxicology and Chemistry, 22, 161–166.
Neuhold, J. M., & Sigler, W. F. (1960). Effects of sodium fluoride on carp and rainbow trout. Transactions of the American Fisheries Society, 89, 358–370.
OECD, Organisation for Economic Co-operation and Development (2010). Detailed review paper (DRP) on mollusks life-cycle toxicity testing. ENV/JM/MONO(2010)9.
Oehlmann, J., & Schulte-Oehlmann, U. (2002). Molluscs as bioindicators. In B. A. Markert, A. M. Breure, & H. G. Zechmeister (Eds.), Bioindicators and biomonitors (pp. 577–635). Amsterdam: Elsevier.
Pedersen, S., Selck, H., Salvito, D., & Forbes, V. (2009). Effects of the polycyclic musk HHCB on individual- and population-level endpoints in Potamopyrgus antipodarum. Ecotoxicology and Environmental Safety, 72, 1190–1199.
Reddy, S. L. N., & Venugopal, N. B. R. K. (1990). Effect of fluoride on acetylcholinesterase activity and oxygen consumption in a freshwater field crab, Barytelphusa guerini. Bulletin of Environmental Contamination and Toxicology, 45, 760–766.
Reddy, S. L. N., Gopal, N., Reddy, A., & Rao, J. (1989). Fluoride-induced changes in carbohydrate metabolism in the tissue of freshwater crab Barytelphusa guereni. Ecotoxicology and Environmental Safety, 18, 59–67.
Rosenberg, D. M., & Resh, V. H. (1993). Freshwater biomonitoring and benthic macroinvertebrates. London: Chapman & Hall. 504 p.
Sigler, W. F., & Neuhold, J. M. (1972). Fluoride intoxication in fish: A review. Journal of Wildlife Diseases, 8, 252–254.
Tripathi, N., Bajpai, S., & Tripathi, M. (2009). Genotoxic alterations induced by fluoride in Asian cat fish, Clarias batrachus (Linn.). Fluoride, 42, 292–296.
U.S. Environmental Protection Agency. (1991). Multifactor probit analysis. EPA 600/X-91-101. Washington, DC: Environmental Protection Agency.
U.S. Environmental Protection Agency. (2002). Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. EPA 821-R-02-013 (4th ed.). Washington, DC: Environmental Protection Agency.
Valenti, T. W., Cherry, D. S., Currie, R. J., Neves, R. J., Jones, J. W., Mair, R., et al. (2006). Chlorine toxicity to early life stages of freshwater mussels (Bivalvia: Unionidae). Environmental Toxicology and Chemistry, 25, 2512–2518.
Wang, N., Ingersoll, C. G., Greer, I. E., Hardesty, D. K., Ivey, C. D., Kunz, J. L., et al. (2007). Chronic toxicity of copper and ammonia to juvenile freshwater mussels (Unionidae). Environmental Toxicology and Chemistry, 26, 2048–2056.
Winterbourn, M. J. (1970). The New Zealand species of Potamopyrgus (Gastropoda: Hydrobiidae). Malacologia, 10, 283–321.
World Health Organization. (2002). Environmental Health Criteria 227. Fluorides. Geneva: WHO.
Acknowledgements
Funds for this research came from the Spanish Ministry of Science and Innovation (CGL2006-06804/BOS). The University of Alcalá provided logistical support. Dr. Álvaro Alonso is currently supported by a Juan de la Cierva contract from the Spanish Ministry of Science and Innovation and by a grant from the Wageningen Institute for Environment and Climate Research (WIMEK) to perform a stay at the Aquatic Ecology and Water Quality Management Group (Wageningen University, The Netherlands). We want to give our sincere gratitude to Dr. Pilar Castro for her comments and suggestions during the writing of the manuscript. Special thanks to Dr. Marieke De Lange and Dr. Edwin Peeters for their support during the stay of A. Alonso at the University of Wageningen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alonso, Á., Camargo, J.A. Toxic Effects of Fluoride Ion on Survival, Reproduction and Behaviour of the Aquatic Snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Water Air Soil Pollut 219, 81–90 (2011). https://doi.org/10.1007/s11270-010-0685-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0685-5