Abstract
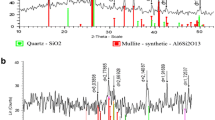
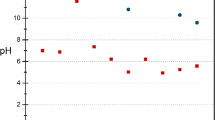
Fly ash generated from medical waste incinerator and wastewater produced from electroplating plants contains various hazardous contaminants such as heavy metals and chlorinated organic compounds. The primary goal of this research was to investigate the feasibility of removing heavy metals from wastewater using medical waste incinerator fly ash as the treatment reagent with addition of small amount of sodium carbonate (Na2CO3) in a hydrothermal process. Copper (Cu) was used as the model heavy metal contaminant in the process. The results revealed that medical waste incinerator fly ash could effectively stabilize Cu(II) ion from wastewater, the crystal phase and simple substance formed during the treatment played a significant role in the fixation of heavy metals in wastewater and fly ash. The heavy metal leachability of treated ash was also measured after removal process. The co-disposal of Cu-containing wastewater and heavy metals-bearing medical waste incinerator fly ash by hydrothermal treatment with addition of a small amount of Na2SO3 was found promising as an effective way of removing Cu from wastewater. The reutilization feasibility of fly ash and the formation mechanism of copper-containing substances were also discussed in this paper.







Similar content being viewed by others
References
Barbieri, L., Karamanov, A., Corradi, A., Lancellotti, I., Pelino, M., & Ma, J. (2008). Rincon structure, chemical durability and crystallization behavior of incinerator-based glassy systems. Journal of Non-crystalline Solids, 354, 521–528.
Bayuseno, A. P., Schmahl, W. W., & Müllejans, T. (2009). Hydrothermal processing of MSWI fly ash-towards new stable minerals and fixation of heavy metals. Journal of Hazardous Materials, 167(1–3), 250–9.
Collivignarelli, C., & Sorlini, S. (2002). Reuse of municipal solid wastes incineration fly ashes in concrete mixtures. Waste Management, 22, 909–912.
Eighmy, T. T., Eusden, J. D., Krzanowski, J. E., Dominggo, D. S., Stampfli, D., Martin, J. R., et al. (1995). Comprehensive approach toward understanding element speciation and leaching behaviour in municipal solid waste incineration electrostatic precipitator ash. Environmental Science and Technology, 29, 629–646.
Esaki, M., Kawakami, I., & Sumitomo, M. (1995). Immobilization of fly ash with cement solidification and chemical treatment. In: Proc. of 6th Annu. Conf. of Japan Society of Waste Management Experts, 432–434.
Gao, X. B., Wang, W., Ye, T., Wang, F., & Lan, Y. X. (2008). Utilization of washed MSWI fly ash as partial cement substitute with the addition of dithiocarbamic chelate. Journal of Environmental Management, 88, 293–299.
GB5085.3. (2007). People's Republic of China national standards, identification standards for hazardous wastes-identification for extraction toxicity.
Gutman, R. (1995). Recycling of municipal solid waste incinerator ash by thermal separation and vitrification. In: Proc. of R ’95 Conf. on Recovery, Recycling and Reintegrating, EMPA, Switzerland; IV, 9–11.
Hearn, B., Michael, R., Hunt, & Hayward, A. (1969). Solubility of cupric oxide in pure subcritical and supercritical water. Journal of Chemical and Engineering Data, 14 (4), 442–447.
HJ/T299. (2007). People's Republic of China environmental protection industry standard, solid waste-extraction procedure for leaching toxicity-sulphuric acid and nitric acid method.
Huang, W. J., & Chu, S. C. (2003). A study on the cementlike properties of municipal waste incineration ashes. Cement and Concrete Research, 33, 1795–1799.
Kalyani, S., Rao, P. S., & Krishnaiah, A. (2004). Removal of nickel (II) from aqueous solutions using marine macroalgae as the sorbing biomass. Chemosphere, 57, 1225–1229.
Karidakis, T., Leonardou, S. A., & Syngouna, P. N. (2005). Removal of magnesium from nickel laterite leach liquors by chemical precipitation using calcium hydroxide and the potential use of the precipitate as a filler material. Hydrometallurgy, 76, 105–114.
Keane, M. A. (1997). The removal of copper and nickel from aqueous solution using Y zeolite ion exchangers. Physicochemical and Engineering Aspects, 138, 11–20.
Kersch, C., Geert, F. W., & Geert, J. W. (2004). Supercritical fluid extraction of heavy metals from fly ash. Industrial & Engineering Chemistry Research, 43, 190–196.
Leusbrocka, I., Sybrand, M. J., Rexwinkel, G., & Geert, F. V. (2008). Quantitative approaches for the description of solubilities of inorganic compounds in near-critical and supercritical water. The Journal of Supercritical Fluids, 47, 117–127.
Michael, D., Morrish, R., & Anthony, J. M. (2008). Kinetics and mechanism for the reaction of hexafluoroacetylacetone with CuO in supercritical carbon dioxide. Journal of the American Chemical Society, 130(49), 16659–16668.
Park, Y. J., & Heo, J. (2002). Vitrification of fly ash from municipal solid waste incinerator. Journal of Hazardous Materials, B91, 83–93.
Rémond, S., Bentz, D. P., & Pimienta, P. (2002). Effects of the incorporation of municipal solid waste incineration fly ash in cement pastes and mortars II: Modeling. Cement and Concrete Research, 32, 565–576.
Rincon, J. M. A., Romero, M., & Boccaccini, A. R. (1999). Microstructural characterisation of a glass and a glass-ceramic obtained frommunicipal incinerator fly ash. Journal of Materials Science, 34, 4413–4423.
Romero, M., Rawlings, R. D., & Rincon, J. M. (1999). Development of a new glass-ceramic by means of controlled vitrification and crystallisation of inorganic wastes from urban incineration. Journal of the European Ceramic Society, 19, 2049–2058.
Susak, N. J., & Crerar, D. A. (1985). Spectra and coordination changes of transition metals in hydrothermal solutions: Implications for ore genesis. Geochimica et Cosmochimica Acta, 49, 555–564.
Tayler, H. F. W. (1990). Cement chemistry (pp. 386–387). London: Academic.
Vasilenko, G. V., Zarembo, V. I., & Slobodov, A. A. (1997). Fundamental aspects of deposition of copper compounds in supercritical pressure power unit turbine. Russian Journal of Applied Chemistry, 70, 1498–1500.
Weirich, D. B., Hari, R., Xue, H., Behra, P., & Sigg, L. (2002). Adsorption of Cu, Cd, and Ni on goethite in the presence of natural groundwater ligands. Environmental Science and Technology, 36, 328–336.
Yamaguchi, H., Shibuya, E., Kanamaru, Y., & Uyama, K. (1996). Hydrothermal decomposition of PCDDs/PCDFs in MSWI fly ash. Chemosphere, 32(1), 203–208.
Yu, J., & Savage, P. E. (2001). Catalyst activity, stability, and transformations during oxidation in supercritical water. Applied Catalysis B: Environmental, 31, 123–132.
Zhang, F. S., & Itoh, H. (2006). Extraction of metals from municipal solid waste incinerator fly ash by hydrothermal process. Journal of Hazardous Materials, B136, 663–670.
Zhang, Y. M., Sun, W., & Yan, H. D. (2000). Hydration of high-volume fly ash cement pastes. Cement & Concrete Composites, 22, 445–452.
Acknowledgment
This project was supported by Program for New Century Excellent Talents in University, National Natural Science Foundation of China (50576082), National High Technology Research and Development Program of China (2007AA06Z336, 2007AA061302), and the Program of Introducing Talents of Discipline to University of China (B08026). We thank Dr. Lee Chun-Wai of US EPA for his comments and the English proof-reading on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, J., Li, X., Chi, Y. et al. Co-disposal of Heavy Metals Containing Waste Water and Medical Waste Incinerator Fly Ash by Hydrothermal Process with Addition of Sodium Carbonate: A Case Study on Cu(II) Removal. Water Air Soil Pollut 209, 391–400 (2010). https://doi.org/10.1007/s11270-009-0207-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0207-5