Abstract
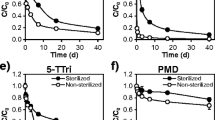
Results from a multi-year, pilot-scale land treatment project for PAHs and PCBs biodegradation were evaluated. A mathematical model, capable of describing sorption, sequestration, and biodegradation in soil/water systems, is applied to interpret the efficacy of a sequential active–passive biotreatment process of organic chemicals on remediation sites. To account for the recalcitrance of PAHs and PCBs in soils and sludges during long-term biotreatment, this model comprises a kinetic equation for organic chemical intraparticle sequestration process. Model responses were verified by comparison to measurements of biodegradation of PAHs and PCBs in land treatment units; a favorable match was found between them. Model simulations were performed to predict on-going biodegradation behavior of PAHs and PCBs in land treatment units. Simulation results indicate that complete biostabilization will be achieved when the concentration of reversibly sorbed chemical (S RA) reduces to undetectable levels, with a certain amount of irreversibly sequestrated residual chemical (S IA) remaining within the soil particle solid phase. The residual fraction (S IA) tends to lose its original chemical and biological activity, and hence, is much less available, toxic, and mobile than the “free” compounds. Therefore, little or no PAHs and PCBs will leach from the treatment site and constitutes no threat to human health or the environment. Biotreatment of PAHs and PCBs can be terminated accordingly. Results from the pilot-scale testing data and model calculations also suggest that a significant fraction (10–30%) of high-molecular-weight PAHs and PCBs could be sequestrated and become unavailable for biodegradation. Bioavailability (large K d , i.e., slow desorption rate) is the key factor limiting the PAHs degradation. However, both bioavailability and bioactivity (K in Monod kinetics, i.e., number of microbes, nutrients, and electron acceptor, etc.) regulate PCBs biodegradation. The sequential active–passive biotreatment can be a cost-effective approach for remediation of highly hydrophobic organic contaminants. The mathematical model proposed here would be useful in the design and operation of such organic chemical biodegradation processes on remediation sites.










Similar content being viewed by others
References
Adeel, Z., Luthy, R. G., Dzombak, D. A., Roy, S. B., & Smith, J. R. (1997). Leaching of PCB compounds from untreated and biotreated sludge-soil mixtures. Journal of Contaminant Hydrology, 28, 289–309.
Alcoa (1994). Evaluation of In Situ Bioremediation Treatment of 60-Acre Lagoon Sludge Material for PCB Reduction/Immobilization at Alcoa’s Massena, NY Facility. Internal report, EHS Technology Center, Alcoa Technical Center, Alcoa Center, Pennsylvania.
Alcoa Remediation Projects Organization (1995). Bioremediation tests for Massena lagoon sludges/sediments. Report prepared for US EPA and New York State Department of Environmental Conservation.
Alexander, M. (1995). How toxic are toxic chemicals in soil? Environmental Science & Technology, 29, 2713–2717.
Alexander, M. (1977). Introduction to soil microbiology (2nd edn.). New York: Wiley.
ASTM (1996). 1996 Annual book of ASTM standards. West Conshohocken, PA: American Society for Testing and Materials.
Ball, W. P., Euehler, E., & Harmon, T. C. (1990). Characterization of a sandy aquifer material at the grain scale. Journal of Contaminant Hydrology, 5, 253–295.
Ball, W. P., & Roberts, V. (1991a). Long-term sorption of halogenated organic chemicals by aquifer material. 1. Equilibrium. Environmental Science and Technology, 25(7), 1223–1237.
Blake, G. R., & Hartge, K. H. (1986). Bulk density. In A. Klute (Ed.), Methods of soil analysis part 1: Physical and mineralogical methods (2nd edn., pp. 363–375). Agronomy Monograph Number 9. Soil Science Society of America, Madison, Wisconsin.
Bollag, J. M., & Loll, M. J. (1983). Incorporation of xenobiotics into soil humus. Experientia, 39, 1221–1231.
Brunauer, S., Emett, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60, 309–319.
Chiou, C. T., Porter, P. E., & Schmedding, D. W. (1983). Partition equilibria of nonionic organic compounds between soil organic matter and water. Environmental Science & Technology, 17, 227–231.
Curtis, G. P., Roberts, P. V., & Reinhard, M. (1986). A natural gradient experiment and solute transport in a sand aquifer: 4. Sorption of organic solutes and its influence on mobility. Water Resources Research, 22, 2059–2067.
Danielson, R. E., & Sutherland, P. L. (1986). Porosity. In A. Klute (Ed.), Methods of soil analysis part 1: Physical and mineralogical methods (2nd edn., pp. 443–461). Agronomy Monograph Number 9. Soil Science Society of America, Madison, Wisconsin.
Focht, D. D., & Brunner, W. W. (1985). Kinetics of biphenyl and polychlorinated biphenyl metabolism in soil. Applied and Environmental Microbiology, 50(10), 1058–1063.
Hayduk, W., & Laudie, H. (1974). Prediction of diffusion coefficients for non-electrolytes in dilute aqueous solutions. AIChE Journal, 20, 611–615.
Hill, M. C. (1998). Methods and guidelines for effective model calibration: US Geological Survey Water- Resources Investigations Report 98-4005, 90 p. 4–32.
Karickhoff, S. W., Brown, D. S., & Scott, T. A. (1979). Sorption of hydrophobic pollutants in sediment suspensions. Water Research, 13(3), 241–248.
Kilbertus, G. (1980). Etudes des microhabitats contenus dans les agregats du sol. Leur relation avec la biomasse bacterienne presente et la taille des procaryotes. Revue d’écologie et de biologie du sol, 17, 543–557.
Kuhn, E. P., Colberg, P. J., Schnoor, J. L., Wanner, O., Zehnder, A. J. B., & Schwarzenback, R. P. (1985). Microbial transformations of substituted benzenes during infiltration of river water to groundwater: Laboratory column studies. Environmental Science & Technology, 19, 961–968.
Kyuma, K., & Kamaguchi, K. (1964). Oxidative changes of polyphenols as influenced by allophane. Soil Science Society of American Journal, 28, 371–374.
Law, A. T., & Button, D. K. (1977). Multiple-carbon-source-limited growth kinetics of a marine coryneform bacterium. Journal of Bacteriology, 129, 115–123.
Lawrence, G. P., Payne, D., & Greenland, D. (1979). Pore size distribution in critical point and freeze dried aggregates from clay subsoils. Journal of Soil Science, 30, 499–516.
Lichtenstein, E. P., De Pew, L. J., Eshbaugh, E. L., & Sleesman, J. P. (1960). Persistence of DDT, aldrin and lindane in some midwestern soils. Journal of Economic Entomology, 53, 136–142.
Linz, D., & Nakles, D. (Eds.) (1997). Environmental acceptable endpoints in soils. Annapolis, MD: American Academy of Environmental Engineers.
Loehr, R. C., & Webster, M. T. (1997). Effect of treatment on contaminant availability, mobility and toxicity. Chapter 2. In D. Linz & D. Nakles (Eds.), Environmentally acceptable endpoints in soils. Annapolis, MD: American Academy of Environmental Engineers.
Luthy, R. G., Aiken, G. R., Brusseau, M. L., Cunningham, S. D., Gschwend, P. M., Pignatello, J. J., et al. (1997). Sequestration of hydrophobic organic contaminants by geosorbents. Environmental Science & Technology, 131, 3341–3347.
Nash, R. G., & Woolson, A. E. (1967). Resistance of chlorinated hydrocarbon insecticides in soil. Science, 157, 924–927.
NATO (1997). Perspective in bioremediation: Technologies for environmental improvement. NATO advanced research workshop on biotechnological remediation of contaminated sites. Dordrecht ,The Netherlands: Kluwer.
NRC (National Research Council) (1993). In situ bioremediation: When does it work? Washington, DC: National Academy Press.
NRC (National Research Council) (1994). Alternatives for ground water cleanup. Washington, DC: National Academy Press.
NYSDEC (1989). New York State Department of Environmental Conservation, Analytical Service Protocol. NYSDEC Document 0073. NYSDEC, Albany, NY.
Schmidt, S. K., & Alexander, M. (1985). Effects of dissolved organic carbon and second substrates on the biodegradation of organic compounds at low concentrations. Applied and Environmental Microbiology, 49(4), 822–827.
Sjoblad, R. D., & Bollag, J. M. (1981). Oxidative coupling of aromatic compounds by enzymes from soil microorganisms. In E. A. Paul & N. N. Ladd (Eds.), Soil biochemistry (pp. 113–152). New York: Marcel Dekker.
Smith, J. R., Tomicek, R. M., Swallow, P. V., Weightman, R. L., Nakles, D. V., & Hebling, M. (1995). Definition of Biodegradation Endpoints for PAH Contaminated Soils Using a Risk-Based Approach. In P. T. Kostecki, E. J. Calabrese & M. Bonazountas (Eds.), Hydrocarbon contaminated soils, vol. V (pp. 521–572). Amherst, MA: Amherst Scientific Publishers.
Statistical Analysis System (SAS) (1988). SAS/ETS Users guide. Version 6, first edition. SAS Institute Inc., Cary, NC.
Tindall, J. A., Kunkel, J. R., & Anderson, D. E. (1999). Unsaturated zone hydrology for scientists and engineers (624 p.). Upper Saddle River, New Jersey: Prentice Hall.
US EPA (1995). Symposium on bioremediation of hazardous wastes. US Environmental Protection Agency, EPA/600/R-95/076. Washington, DC: US EPA.
US Environmental Protection Agency (1983). Methods for chemical analysis of water and wastes. US EPA, environmental monitoring and support laboratory. US EPA rep. EPA-600/4-79-020. US Gov.print. office, Washington, DC.
US Environmental Protection Agency (1995). Methods for evaluating solid waste: Phsical/chemical methods (SW-846), third edn. January, 1995. US EPA, Washington, DC.
Weber, W. J. Jr., & DiGiano, F. A. (1996). Process dynamics in environmental systems. A wiley-interscience publication. New York: Wiley.
Zhang, W. -X., & Bouwer, E. J. (1997). Biodegradation of benzene, toluene and naphthalene in soil-water-slurry microcosms. Biodegradation, 8, 167–175.
Acknowledgments
This work is supported by the National Natural Science Foundation of China under contract no. 59909003. The authors would also like to acknowledge Alcoa Inc. (Pittsburgh, PA) for technical support in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, L., Tindall, J.A. & Friedel, M.J. Biodegradation of PAHs and PCBs in Soils and Sludges. Water Air Soil Pollut 181, 281–296 (2007). https://doi.org/10.1007/s11270-006-9299-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-006-9299-3