Abstract
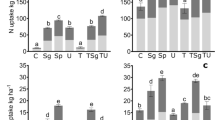
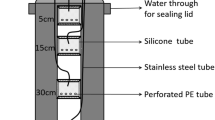
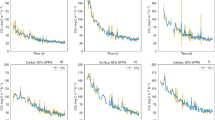
We examined the influence of various urea granule sizes (< 2, 7.0, 9.9 and 12.7 mm) applied into a silt loam soil (experiment 1) and soil types (sandy, silt and clay loam) treated with the largest granule (experiment 2) on gaseous N loss (except N2) at field capacity. The prilled urea (PU) was mixed into the soil whereas the urea granules were point-placed at a 5.0-cm depth. For experiment 1, N2O emission was enhanced with increasing granule size, ranging from 0.17–0.50% of the added N during the 45-day incubation period. In the case of experiment 2, the sandy loam soil (0.59%) behaved similarly with the silt loam (0.53%) but both showed remarkably lower emissions than were found for the clay loam soil (2.61%). Both nitrification and N2O emissions were delayed by several days with increasing granule size, and the latter was influenced by mineral N, soil water and pH. By contrast, the NH3 volatilization decreased with increasing granule size, implying the inhibition of urease activity by urea concentration gradients. Considering both experimental results, the NH3 loss was highest for the PU-treated (1.73%) and the larger granules regardless of soil type did not emit more than 0.27% of the added N over 22 days, possibly because the high concentrations of either mineral N or NH4 + in the soil surface layer (0–2.5 cm) and the high H+ buffering capacity might regulate the NH3 emission. Similar to the pattern of NH3 loss, NOx emission was noticeably higher for the PU-treated soil (0.97%) than for the larger granule sizes (0.09–0.29%), which were the highest for the sandy and clay loam soils. Positional differences in the concentration of mineral N and nitrification also influenced the NOx emission. As such, total NH3 loss was proportional to total NOx emission, indicating similar influence of soil and environmental conditions on both. Pooled total N2O, NH3 and NOx emission data suggest that the PU-treated soil could induce greater gaseous N loss over larger urea granules, largely in the form of NH3 and NOx emissions, whereas a similar increase with the largest granule size was mainly due to the total N2O flux.
Similar content being viewed by others
References
Bouwman, A.F., Boumans, L.J.M., & Batjes, N.H. (2002). Estimation of global ammonia volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Global Biogeochemical Cycles, 116, DOI 10.1029/2000GB001389.
Bouwmeester, R.J.B., Vlek, P.L.G., & Stumpe, J.M. (1985). Effect of environmental factors on ammonia volatilization from a urea-fertilized soil. Soil Science Society of American Journal, 49, 376–381.
Bremner, J.M. (1997). Sources of nitrous oxide in soils. Nutrition Cycle in Agroecosystems, 49, 7–16.
Buresh, R.J. (1987). Ammonia volatilization from point-placed urea in upland, sandy soils. Fertilizer Research, 12, 263–268.
Crutjen, P.J. (1979). The role of NO and NO2 in the chemistry of the troposphere and stratosphere. Annual Review Earth and Planet Science, 7, 442–472.
Davidson, E.A. (1993). Soil water content and the ratio of nitrous oxide to nitric oxide emitted from soil. In R. S. Oremland (Ed.), Biogeochemistry of global change: Radiatively active trace gases (pp. 369–386). New York: Chapman and Hall.
Delmas, R., Serça, D., & Jambert, C. (1997). Global inventory of NOrm x sources. Nutrition Cycle in Agroecosystems, 48, 51–60.
Dobbie, K.E., McTaggart, L.P., & Smith, K.A. (1999). Nitrous oxide emission from intensive agricultural system: Variations between crops and seasons, key driving variables, and mean emission factors. Journal of Geophysical Research, 104, 26891–26899.
Eichner, M.J. (1990). Nitrous oxide emissions from fertilized soils: summary of available data. Journal of the Environmental. Quality, 19, 272–280.
Ferguson, R.B., & Kissel D.E. (1986). Effects of soil drying on ammonia volatilization from surface-applied urea. Soil Science Society of American Journal, 50, 485–490.
Ferguson, R.B., Kissel, D.E., Koelliker, J.K., & Basel, W. (1984). Ammonia volatilization from surface-applied urea: effect of hydrogen ion buffering capacity. Soil Science Society of American Journal, 48, 578–582.
Firestone, M.K., & Davidson, E.A. (1989). Microbiological basis of NO and N2O production and consumption. In M. O. Andreae, & D. S. Schimel (Eds.), Exchange of trace gases between terrestrial ecosystems and the atmosphere (pp. 7–21). Chichester: John Wiley.
Haque, S.A. (2002). Urea super granule point placement on potato: a new concept. In Proceedings of the 17th World Congress of Soil Science. 14–21 August, 2002, Thailand, Symposium No. 14. 2030, pp. 1–6.
Hosen, Y., Paisancharoen, K., & Tsuruta, H. (2002). Effects of deep application of urea on NO and N2O emissions from an Andisol. Nutrition Cycle in Agroecosystems, 63, 197–206.
Hou, A.X., & Tsuruta, H. (2003). Nitrous oxide and nitric oxide fluxes from an upland field in Japan: effect of urea type, placement, and crop residues. Nutrition Cycle in Agroecosystems, 65, 191–200.
Hutchinson, G.L., & Brams, E.A. (1992). NO vs. N2O emissions from an NH4 +-amended Bermuda grass pasture. Journal of Geophysical Research, 97, 9889–9896.
Hutchinson, G.L., Vigil, M.F., Doran, J.W., & Kessavalou, A. (1997). Modeling the net microbial production and the soil-atmosphere exchange of gaseous NOrm ×. Nutrition Cycle in Agroecosystems, 48, 25–35.
Keeney D.R., & Nelson D.W. (1982). Nitrogen-inorganic forms. In Page A.L., D.R. Keeney, D.F. Baken, R.H. Miller, R. Ellis, & J.P. Rhoades (eds.), Methods of soil analysis, Part 2 (pp. 643–693). Madison, Wis.: American Society of Agro-nomy.
Khalil, M.I., Rosenani, A.B., Van Cleemput, O., Fauziah, C.I., & Shamshuddin, J. (2002a). Nitrous oxide emission from a maize-groundnut crop rotation supplied with different nitrogen sources in the humid tropics. Journal of Environment Quality, 31, 1071–1078.
Khalil, M.I., Rosenani, A.B., Van Cleemput, O., Shamshuddin, J., & Fauziah, C.I. (2002b). Nitrous oxide production from an ultisol treated with different nitrogen sources and moisture regimes. Biological and Fertility of Soils, 36, 59–65.
Khalil, M.I., & Baggs, E.M. (2005). CH4 oxidation and N2O emissions at varied soil water-filled pore spaces and headspace CH4 concentrations. Soil Biological and Biochemistry, 37, 1785–1794.
Khalil, M.I., Schmidhalter, U., & Gutser, R. (2005). Urea super granules in a cambisol: N transformations, N2O and NH3 emissions at two soil water regimes. In C. J. Li et al. (Eds.), Plant nutrition for food security, human health and environmental protection (pp. 1122–1123). China: Tsinghua University Press.
Kuzyakov, Y, Friedel, J.K., & Stahr, K. (2000). Review and mechanisms and quantification of priming effects. Soil Biological and Biochemistry, 32, 1485–1498.
Mohanty, S.K., Singh, U., Balasubramanian, V., & Jha, K.P. (1999). Nitrogen deep-placement technologies for productivity, profitability, and environmental quality of rainfed lowland systems. Nutrition Cycle in Agroecosystems, 53, 43–57.
Mosier A., Kroeze, C., Nevison, C., Oenema, O., Seitzinger, S., & Van Cleemput, O. (1998). Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. Nutrition Cycle in Agroecosystems, 52, 225–248.
Mulvaney, R.L., Khan, S.A., & Mulvaney, C.S. (1997). Nitrogen fertilizers promote denitrification. Biological and Fertility of Soils, 24, 211–220.
Patel, S.K., Panda, D., & Mohanty, S.K. (1989). Relative ammonia loss from urea-based fertilizers applied to rice under different hydrological situations. Fertilizer Research, 19, 113–119.
Remde, A., & Conrad, R. (1990). Production of nitric oxide in Nitrosomonas europaea by reduction of nitrite. Archives Microbiology, 154, 187–191.
Remde, A., Slemr, F., & Conrad, R. (1989). Microbial production and uptake of nitric oxide in soil. FEMS Microbiology Ecology, 62, 221–230.
Russow, R., Sich, I., & Neue, H.-U. (2000). The formation of the trace gases NO and N2O in soils by the coupled process of nitrification and denitrification: results of kinetic 15N tracer investigations. Chemosphere — Global Change Biology, 2, 359–366.
Savant, S.K., & Stangel, P.J. (1990). Deep placement of urea super granules in transplanted rice: principles and practices. Fertilizer Research, 25, 1–83.
Schulze, E.-D., De Vries, W., Hauhs, M., Rosén, K., Rasmussen, L., Tamm, C.-O. and Nilsson, J. (1989). Critical loads for nitrogen deposition on forest ecosystems. Water, Air, Soil Pollution, 48, 451–456.
Shah, S.B., & Wolfe, M.L. (2003). Particle size impacts of subsurface-banded urea on nitrogen transformation in the laboratory. Communication Soil Science and Plant Analysis, 34, 1245–1260.
Singh, Y., Malhi, S.S., Nyborg, M., & Beauchamp, E.G. (1994). Large granules, nests or bands: Methods of increasing efficiency of fall-applied urea for small cereal grains in North America. Fertilizer Research, 38, 61–87.
Sinowski, W., & Auerswald, K. (1999). Using relief parameters in a discriminant analysis to stratify geological areas of different spatial variability of soil properties. Geoderma, 89, 113–128.
Skiba, U., Fowler, D., & Smith, K.A. (1997). Nitric oxide emissions from agricultural soils in temperate and tropical climates: sources, controls and mitigation options. Nutrition Cycle in Agroecosystems, 48, 139–153.
Smith, K.A., McTaggart, I.P., Dobbie, K.E., & Conen, F. (1998). Emissions of N2O from Scottish agricultural soils, as a function of fertilizer N., Nutriton Cycle Agroecosystems, 52, 123–130.
Sommer, S.G., Scjoerring, J.K., & Denmead, O.T. (2004). Ammonia emission from mineral fertilizers and fertilized crops. Advanced Agronomy, 82, 557–622.
Sowers, T. (2001). N2O record spanning the penultimate deglaciation from the Vostok ice core. Journal of Geophysical Research, 160, 31, 903–31, 914.
Tenuta, M., & Beauchamp, E.G. (2000). Nitrous oxide production from granules of different sizes. Journal of Environmental Quality, 29, 1408–1413.
Tenuta, M., & Beauchamp, E.G. (2003). Nitrous oxide production from granular nitrogen fertilizers applied to a silt loam soil. Canadian Journal of Soil Science, 83, 521–532.
Van Cleemput, O. (1998). Subsoils: Chemo- and biological denitrification, N2O and N2 emissions. Nutrition Cycle in Agroecosystems, 52, 187–194.
Vilsmeier, K. (1984). Bestimmung von Dicyandiamid, Nitrit und Nitrat in Bodenextrakten mit HochdruckflÜssigkeitschro-matographie. Zeitshrift fü Pflanzenernahrung und Bodenkunde,, 147, 264–268.
Wang, Z-H., Liu, X-J., Ju, X-T., Zhang, F-S., & Malhi, S.S. (2004). Ammonia volatilization loss from surface-broadcast urea: comparison of vented- and closed-chamber methods and loss in winter wheat-summer maize rotation in North China Plain. Communication in Soil Science and Plant Analysis, 35, 2917–2939.
Weber, A., Gutser, R., & Schmidhalter, U. (2001). Field emissions of NH3 and NOrm x following urea application to wheat. In W. J. Horst et al. (Eds.), Plant nutrition — food security and sustainability of agro-ecosystems (pp. 884–885). The Netherlands: Kluwer Academic Publishers.
Wrage, N., Velthof, G.L., Landbroek, H.J., & Oenema, O. (2004). Nitrous oxide production in grassland soils: assessing the contribution of nitrifier denitrification. Soil Biology and Biochemistry, 36, 229–236.
Yienger, J.J., & Levy II, H. (1995). Empirical model of the global soil-biogenic NOrm x emissions. Journal of Geophysical Research., 100, 11447–11464.
Zheng, X., Huang, Y., Wang, Y., Wang, M., Jin, J., & Li, L. (2003). Effects of soil temperature on nitric oxide emission from a typical Chinese rice-wheat rotation during the non-waterlogged period. Global Change Biological, 9, 601–611.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11270-006-9260-5
Rights and permissions
About this article
Cite this article
Khalil, M.I., Schmidhalter, U. & Gutser, R. N2O, NH3 and NOx Emissions as a Function of Urea Granule Size and Soil Type Under Aerobic Conditions. Water Air Soil Pollut 175, 127–148 (2006). https://doi.org/10.1007/s11270-006-9117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-006-9117-y