Abstract
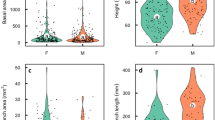
In dioecious species, females usually have higher sex-related costs than males. Consequently, trade-offs involving survival, growth, and reproduction are expected to differ between sexes. Here, we investigate several aspects of sex-related costs to test whether they are higher for females than males of a widely distributed, locally abundant, dioecious tropical forest tree species. For this, every adult of Mollinedia schottiana (Spreng.) Perkins (Monimiaceae) had its stem diameter at soil height (DSH) and spatial location measured in two 1-ha plots located at the Atlantic Rainforest, SE Brazil. Flowering phenology was also recorded over 12 months. At a second population census, the surviving individuals from the first census had their DSH remeasured. In comparison to males, females did not flower less frequently, less intensely, or in a lower proportion over 12 months. They also did not grow less between censuses, have larger DSH, or show spatial segregation from males. However, sex ratio was male biased, which, together with floral biology, is likely a strategy of M. schottiana to pollination by thrips. This study shows that dioecious species do not necessarily have differential sex-related costs as expected by the higher investment in reproductive structures by females. Sex ratios, which are often interpreted as a result of sex-related costs, can be driven by the reproductive biology of plant species.



Similar content being viewed by others
References
Bagchi R, Henrys PA, Brown PE, Burslem DFRP, Diggle PJ, Gunatilleke CVS, Gunatilleke IAUN, Kassim AR, Law R, Noor S, Valencia RL (2011) Spatial patterns reveal negative density dependence and habitat associations in tropical trees. Ecology 92:1723–1729. https://doi.org/10.1890/11-0335.1
Bierzychudek P, Eckhart V (1988) Spatial segregation of the sexes of dioecious plants. Am Nat 132:34–43. https://doi.org/10.1086/284836
Buchmann T, Schumacher J, Roscher C (2018) Intraspecific trait variation in three common grass species reveals fine-scale species adjustment to local environmental conditions. J Plant Ecol 11:887–898. https://doi.org/10.1093/jpe/rtx068
Bullock SH, Bawa KS (1981) Sexual dimorphism and the annual flowering pattern in Jacaratia dolichaula (D. Smith) woodson (caricaceae) in a costa rican rain forest. Ecology 62:1494–1504. https://doi.org/10.2307/1941506
Caetano APS, Teixeira SP, Forni-Martins ER, Carmello-Guerreiro SM (2013) Pollen insights into apomitic and sexual Miconia (Miconiae, Melastomataceae). Int J Plant Sci 174:760–768. https://doi.org/10.1086/669927
Carman JG (1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, biposry, tetraspory, and polyembriony. Biol J Linn Soc 61:51–94. https://doi.org/10.1111/j.1095-8312.1997.tb01778.x
Chave J, Muller-Landau HC, Baker TR, Easdale TA, ter Steege H, Webb CO (2006) Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol Appl 16:2356–2367. https://doi.org/10.1890/1051-0761(2006)016[2356:RAPVOW]2.0.CO;2
Chazdon RL, Careaga S, Webb C, Vargas O (2003) Community and phylogenetic structure of reproductive traits of woody species in wet tropical forests. Ecol Monogr 73:331–348. https://doi.org/10.1890/02-4037
Ciiagro (2017) Centro integrado de informações agrometereológicas. http://www.ciiagro.sp.gov.br. Accessed 20 Aug 2017
Cipollini ML, Stiles E (1991) Costs of reproduction in Nyssa sylvatica: sexual dimorphism in reproductive frequency and nutrient flux. Oecologia 86:585–593. https://doi.org/10.1007/BF00318326
Endress PK (1979) Noncarpellary pollination and “hyperstigma” in an angiosperm (Tambourissa religiosa, Monimiaceae). Experientia 35:45. https://doi.org/10.1007/BF01917867
Endress PK, Ingersheim A (1997) Gynoecium diversity and systematic of the Laurales. J Linn Soc Bot 125:93–168. https://doi.org/10.1111/j.1095-8339.1997.tb02250.x
Feil JP (1992) Reproductive ecology of dioecious Siparuna (Monimiaceae) in ecuador – a case of gall midge pollination. Bot J Linn Soc 110:171–203. https://doi.org/10.1111/j.1095-8339.1992.tb00290.x
Forero-Montaña J, Zimmerman JK, Thompson J (2010) Population structure, growth rates and spatial distribution of two dioecious tree species in a wet forest in puerto rico. J Trop Ecol 26:433–443. https://doi.org/10.1017/S0266467410000143
Fournier LA (1974) Um método cuantitativo para la medición de características fenológicas em árboles. Turrialba 26:422–423
Freeman DC, Klikoff LG, Harper KT (1976) Differential resource utilization by the sexes of dioecious plants. Science 193:597–599. https://doi.org/10.1126/science.193.4253.597
Freeman DC, McArthur ED, Harper KT (1984) The adaptive significance of sex liability in plants using Atriplex canescens as a principal example. Ann Mo Bot Gard 71:265–277. https://doi.org/10.1006/jare.2000.0692
García MBG, Antor RJ (1995) Sex ratio and sexual dimorphism in the dioecious Borderea pyrenaica (Dioscoreaceae). Oecologia 101:59–67. https://doi.org/10.1007/BF00328901
Gottsberger G (1977) Some aspects of beetle pollination in the evolution of flowering plants. In: Kubitsky K (ed) Plant systematics and evolution, supplementum 1. Springer-Verlag, Berlin, pp 211–226
Joly CA, Assis MA, Bernacci LC, Tamashiro JY, Campos MCR, Gomes JAMA, Lacerda MS, Santos FAM, Pedroni F, Pereira LS, Padgurschi MCG, Prata EMB, Ramos E, Torres RB, Rochelle A, Martins FR, Alves LF, Vieira SA, Martinelli LA, Camargo PB, Aidar MPM, Eisenlohr PV, Simões E, Villani JP, Belinello R (2012) Florística e fitossociologia em parcelas permanentes da mata atlântica do sudeste do Brasil ao longo de um gradiente altitudinal. Biota Neotrop 12:1–23. https://doi.org/10.1590/S1676-06032012000100012
Judd WS, Campbell CS, Kellog EA, Stevens PF, Donoghue MJ (2007) Plant systematics: a phylogenetic approach, 3rd edn. Sinauer Associates, Sunderland
Juvany M, Munné-Bosch S (2015) Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context. J Exp Bot 66:6083–6092. https://doi.org/10.1093/jxb/erv343
Kirk WDJ (1987) How much pollen can thrips destroy? Ecol Entomol 12:31–40. https://doi.org/10.1111/j.1365-2311.1987.tb00982.x
Lloyd DG (1974) Theoretical sex ratios of dioecious and gynodioecious angiosperms. Heredity 31:11–34. https://doi.org/10.1038/hdy.1974.2
Lloyd DG, Webb CJ (1977) Secondary sex characters in plants. Bot Rev 43:177–216. https://doi.org/10.1007/BF02860717
Martins SC, Sousa Neto E, Piccolo MDC, Almeida DQA, Camargo PB, Carmo JB, Porder S, Lins SRM, Martinelli LA (2015) Soil texture and chemical characteristics along an elevation range in the coastal Atlantic forest of Southeast Brazil. Geoderma Regional 5:106–116. https://doi.org/10.1016/j.geodrs.2015.04.005
Martins VF (2011) Padrão espacial de três espécies arbóreas ornitocóricas da Floresta Ombrófila Densa de Terras Baixas no litoral norte do estado de São Paulo. Dissertation. University of Campinas, Campinas
Matsushita M, Takao M, Makita A (2016) Sex-different response in growth traits to resource heterogeneity explains male-biased sex ratio. Acta Oecol 75:8–14. https://doi.org/10.1016/j.actao.2016.06.009
McMahon CR, Bradshaw CJA (2008) Fecundity. In: Jørgensen SE, Fath BD (eds) Population dynamics. Vol. [2] of encyclopedia of ecology. Elsevier, Oxford, pp 1535–1543
Meagher TR (1988) Sex determination in plants. In: Lovett-Doust J, Lovett-Doust L (eds) Plant reproductive ecology: patterns and strategies. Oxford University Press, New York, pp 125–138
Meagher TR, Antonovics J (1982) The population biology of Chamaelirium luteum, a dioecious of the lily family: life history studies. Ecology 63:1690–1700. https://doi.org/10.2307/1940111
Morellato LPC, Haddad CFB (2000) Introduction: the brazilian atlantic forest. Biotropica 32:786–792. https://doi.org/10.1646/0006-3606(2000)032[0786:ITBAF]2.0.CO;2
Nathan R, Muller-Landau HC (2000) Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol 15:278–285. https://doi.org/10.1016/S0169-5347(00)01874-7
Nicotra AB (1998) Sex ratio variation and spatial distribution of Siparuna grandiflora, a tropical dioecious shrub. Oecologia 115:102–113. https://doi.org/10.1007/s004420050496
Nicotra AB (1999) Reproductive allocation and the long-term costs of reproduction in Siparuna grandiflora, a dioecious neotropical shrub. J Ecol 87:138–149. https://doi.org/10.1046/j.1365-2745.1999.00337.x
Nicrota AB (1999) Sexually dimorphic growth in the dioecious tropical shrub, Siparuna grandiflora. Funct Ecol 13:322–331. https://doi.org/10.1046/j.1365-2435.1999.00326.x
Obeso JR (2002) The cost of reproduction in plants. New Phytol 115:321–348. https://doi.org/10.1046/j.1469-8137.2002.00477.x
Peixoto AL (1987) Revisão taxonômica do gênero Mollinedia Ruiz et Pavon (Monimiaceae, Monimioideae). Dissertation. University of Campinas, Campinas
Prinzing A, Durka W, Klotz S, Brandl R (2001) The niche of higher plants: evidence for phylogenetic conservatism. P Roy Soc Lond B Bio 268:S2383–S2389. https://doi.org/10.1098/rspb.2001.1801
Queenborough SA, Burslem DFRP, Garwood NC, Valencia R (2007) Determinants of biased sex ratios and inter-sex costs of reproduction in dioecious tropical forest trees. Am J Bot 94:67–78. https://doi.org/10.3732/ajb.94.1.67
Queenborough SA, Mazer SJ, Vamosi SM, Garwood NC, Valencia R, Freckleton RP (2009) Seed mass, abundance and breeding system among tropical forest species: do dioecious species exhibit compensatory reproduction or abundances? J Ecol 97:555–566. https://doi.org/10.1111/j.1365-2745.2009.01485.x
R Core Team (2017) R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org/. Accessed 18 Sep 2019
Renner SS, Feil JP (1993) Pollination of tropical dioecious angiosperms. Am J Bot 80:1100–1107. https://doi.org/10.1002/j.1537-2197.1993.tb15337.x
Ribeiro KFO (2016) Produção de frutos e padrão espacial de populações de espécies arbóreas tropicais. Dissertation. University of Campinas, Campinas
Romanov MS, Endress PK, Bobrov AVFCH, Melikian AP, Bejerano AP (2007) Fruit structure and systematics of Monimiaceae s.s. (Laurales). Bot J Linn Soc 153:265–285. https://doi.org/10.1111/j.1095-8339.2007.00609.x
Rosa LBG, Sampaio MB, Martins VF (2019) Local spatial variation in the population dynamics of two species in a region of atlantic rainforest, SE Brazil. Braz J Bot 42:671–680. https://doi.org/10.1007/s40415-019-00563-w
Shea MM, Dixon PM, Sharitz RR (1993) Size differences, sex ratio, and spatial distribution of male and female water tupelo, Nyssa aquatica (Nyssaceae). Am J Bot 80:26–30. https://doi.org/10.2307/2445116
Stanton ML (1994) Male-male competition during pollination in plant populations. Am Nat 144:S40–S68. https://doi.org/10.1086/285652
Stoyan D, Penttinen A (2000) Recent applications of point process methods in forestry statistics. Stat Sci 15:61–78. https://doi.org/10.1214/ss/1009212674
Stoyan D, Stoyan H (1994) Fractals, random shapes and point fields. Methods of geometrical statistics. Wiley, Hoboken
Svenning JC (2001) On the role of microenvironmental heterogeneity in the ecology and diversification of neotropical rain-forest palms (Arecaceae). Bot Rev 67:1–53. https://doi.org/10.1007/BF02857848
Totland Ø, Birks HJB (1996) Factors influencing inter-population variation in Ranunculus acris seed production in an alpine area of southwestern norway. Ecography. https://doi.org/10.1111/j.1600-0587.1996.tb01254.x
Thomas SC, LaFrankie JV (1993) Sex, size and interyear variation in flowering among dioecious trees of the malayan rain forest. Ecology 74:1529–1537. https://doi.org/10.2307/1940080
Wiegand T, Moloney KA (2014) Handbook of spatial point pattern analysis in ecology. CRC Press, Boca Raton
Wiegand T, Grabarnik P, Stoyan D (2016) Envelope tests for spatial point patterns with and without simulation. Ecosphere 7:e01365. https://doi.org/10.1002/ecs2.1365
Williams GA, Adam P (1994) A review of rainforest pollination and plant-pollinator interactions, with particular reference to australian subtropical rainforests. Aus Zool 29:177–212. https://doi.org/10.7882/AZ.1994.006
Williams GA, Adam P, Mound LA (2001) Thrips (Thysanoptera) pollination in australian subtropical rainforests, with particular reference to pollination of Wilkiea huegeliana (Monimiaceae). J Nat Hist 35:1–21. https://doi.org/10.1080/002229301447853
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson Prentice Hall, Upper Saddle River
Acknowledgements
We thank RA Montgomery, AS Penha, RAG Viani, and an anonymous reviewer for their valuable comments; ER Forni-Martins and K Agostini for their insights on plant reproductive biology; KFO Ribeiro for help with spatial point pattern analysis. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 (data collection by LBG Rosa), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—PhD scholarship to VF Martins (142295/2006-0) and Edital Universal (459941/2014-3), and grant #2003/12595-7, São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by R. A. Montgomery.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins, V.F., Bispo, R.L.B. & de Paula Loiola, P. A case of gender equality: absence of sex-related costs in a dioecious tropical forest tree species. Plant Ecol 222, 275–288 (2021). https://doi.org/10.1007/s11258-020-01105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-020-01105-1