Abstract
Heterodichogamy, including protandrous (PA) and protogynous (PG) morphs, is considered a mechanism to avoid selfing and promote disassortative mating. Although morphotypes are usually present in a population at a 1:1 ratio, this ratio may be biased in a low-density population by demographic stochasticity, resulting in a deficiency of mating partners in a neighbourhood dominated by a single morph. In this study, we determined morph ratio of the heterodichogamous tree species, Juglans ailantifolia by observing flowering in a low-density population during 2 years. The morph ratio (PG: PA) of 2.56:1 deviated significantly deviated from 1:1. We genotyped 59 reproductive trees and 405 offspring derived from eight PG-mother and three PA-mother trees with 11 microsatellite markers. Paternity analysis was conducted to clarify the effects of mother morph on the proportion of intra-morph mating. Then, we applied the Bayesian mixed effect mating model (MEMM) to clarify mating system, pollen dispersal, and individual fecundity of PG- and PA-mother trees. We found that the selfing rate and the distance of pollen dispersal were not clearly different between PG- and PA-mother trees. In contrast, the proportion of intra-morph mating was higher in the majority-morph (PG) mother trees than in the minor-morph (PA) mother trees. The MEMM indicated that mean dispersal distance of PG-mother trees was larger than that of PA-mother trees with large variance. Furthermore, we observed individuals with unusually high intra-morph fecundity for majority-morph (PG) trees. These findings indicate that intra-morph mating may occur when majority-morph mothers suffer a deficiency of potential inter-morph mates.
Similar content being viewed by others
Introduction
Most angiosperm populations include a single floral morph. Less frequently, plant populations include two or more morphs that promote inter-morph (disassortative) mating, e.g., heterostyly and heterodichogamy. Inter-morph mating is promoted by the reciprocal position of stigmas and anthers in the two morphs (pin and thrum) and by self- and intra-morph mating incompatibility in heterostylous plants. As a temporal analog to heterostyly, heterodichogamy is a polymorphic sexual system in phenology that involves two genetic morphs: protogynous (PG: stigmas are receptive before pollen is shed) and protandrous (PA: pollen is shed before stigmas are receptive) (Renner 2001; Kimura et al. 2003; Bai et al. 2006). Highly polymorphic microsatellite markers revealed that inter-morph mating was prevalent in natural populations of the heterodichogamous plants such as Juglans mandshurica (Bai et al. 2006), Acer opalus (Gleiser et al. 2008a, b), and A. mono (Kikuchi et al. 2009).
Human-mediated disturbances to forest ecosystems, such as habitat fragmentation and selective logging, can affect mating system and pollen dispersal in plant populations. Outcrossing rates are significantly lower in disturbed (small-isolated and/or low-density) areas than that in undisturbed areas in many plant species (Eckert et al. 2010). In contrast, for some plant species, pollen dispersal is more extensive in fragmented and/or low-density areas than in continuous and high-density areas (e.g., White et al. 2002; Bacles and Ennos 2008). Thus, information on mating patterns in disturbed areas is of recent concern.
The effects of disturbances on mating system and pollen dispersal in sexually polymorphic plants are complex. Outcrossing via inter-morph mating is effective and stable at equal morph frequency (Gleeson 1982; Kery et al. 2003). Demographic stochasticity, however, often produces asymmetric deviations from an even morph frequency, particularly in small populations (Kery et al. 2003). In a small, morph-ratio-biased population, females of the majority morphotype will be confronted with a deficiency of mating partners (opposite-morph plants) in their neighbourhood. In contrast, females of the minority morphotype will have an abundance of potential mating partners. Ishihama et al. (2003) assessed seed production and gene flow in a heterostylous perennial species, Primula sieboldii, using a patchy experimental population. In patches containing no opposite-morph genets, seed set was significantly lower than in patches containing opposite-morph genets. In patches without opposite-morph genets, seed were fertilized by pollen from opposite-morph outside the patch or were produced by selfing. Gleiser et al. (2008b) investigated the mating system in a morph-ratio-biased population of the heterodichgamous tree, Acer opalus. They revealed that the selfing rate was similar for majority- and minority-morph mother trees, whereas intra-morph mating was detected only in majority-morph mother trees. These facts indicate that the proportion of seeds produced by (1) selfing, (2) intra-morph mating in the neighbourhood, and (3) inter-morph mating via long-distance pollen dispersal may increase in more sparse and morph-biased populations.
To conserve rare and scattered population of a heterodicogamous plant species, a better understanding of the reproductive dynamics in low-density and/or morph-ratio-biased populations at the local scale is required. In low-density and/or morph-ratio-biased populations, seeds are produced by intra-morph mating, selfing, and/or pollen dispersed over long distances because inter-morph trees might not be sufficiently available in the vicinity of majority-morph mother trees. Under these circumstances, three hypotheses are proposed: (1) The intra-morph mating in majority-morph mother trees is higher than that in minority-morph trees, (2) the proportion of selfing of majority-morph mother trees is higher than that in minority-morph trees, and (3) the average distance of pollen flow of majority-morph mother trees would be greater than that of minority-morph trees.
In this study, we determined the morph ratio of the heterodichogamous tree, Juglans ailantifolia in a low-density population by observing flowering during a 2-year period. Then, seeds were collected from mothers of each morphotype. We performed a paternity analysis with highly polymorphic microsatellilte markers evaluating the effects of mother morph type on the proportion of intra-morph mating. We also applied a Bayesian mixed effect mating model (MEMM) based on Bayesian approach to genotypic data to quantify selfing rate, immigration rate, pollen dispersal distance, and individual fecundity. Finally, the effects of mother morph type on the proportion of intra-morph mating, immigration rate, selfing rate, and pollen dispersal distance were evaluated to test the three proposed hypotheses.
Materials and methods
Study species
Juglans ailantifolia Carr. (Juglandaceae) is a monoecious wind-pollinated deciduous tree, widely distributed from Kyushu to Hokkaido in Japan. This species is a typical heterodichogamous plant, with protogynous and protandrous trees. It has been suggested that the Juglandaceae are entirely self-compatible (Thompson and Romberg 1985; Renner 2001), and this was also found in a self-pollination experiment with J. ailantifolia (Kimura unpublished data).
Study site
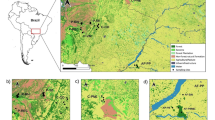
The study site was located in the Iwanazawa Forest Reserve in University Forest in Hokkaido, Graduate School of Agricultural and Life Sciences, the University of Tokyo, in Furano, central Hokkaido, Japan (Lat 43. 10–20′N, 142. 18–40′ 348–411 m elevation). The annual precipitation and mean air temperature at the nearest meteorological station were 983.9 mm and 6.1 ºC in 2002, respectively. The Iwanazawa Forest Reserve was established in 1994. This riparian forest community is dominated by two conifers (Abies sachalinensis and Picea jezoensis) and broad-leaved trees such as Fraxinus mandshurica var. japonica, Acer mono, Alnus hirsuta, Cercidiphyllum japonicum, Ulmus davidiana var. japonica, and Tilia japonica, all of which are common in riparian forests of this region. Until this forest was registered as a forest reserve in 1994, overmature trees were selectively removed every 10 years (Watanabe and Sasaki 1994), possibly reducing the density of J. ailantifolia. Nevertheless, Matsui et al. (2002) indicated that J. ailantifolia produced seeds in the study site, despite the species’ low density (about 0.3 trees per ha). We set a quadrat (approximately 3,000 m × 600 m, 180 ha) that included 59 reproductive trees of J. ailantifolia (Fig. 1). Because most of the J. ailantifolia in this study were growing alongside a stream, and the surroundings riparian zone consists of conifer plantations, J. ailantifolia rarely occurred outside the quadrat.
Distribution of PG trees (closed circle), PA trees (open circle), and female trees (closed triangle) in the study quadrat. Mother ID is shown for all 11 seed-bearing trees in the study area, as well as number of seeds surveyed. Black, light gray, dark gray, and white wedges represent selfing, immigrant pollen dispersal, intra-morph mating, and inter-morph mating, respectively
Flowering phenology and sexual morph identification
Female and male flowering periods were temporally segregated within individual trees and among trees of the same morphotype. Each morphotype’s sexual functions were synchronous and reciprocal with the other morphotype (Kimura et al. 2003). Individuals were classified into three sexual-morph types based on flowering phenology in 2002 and 2003: Protogynous (PG) individuals produced receptive female flowers before the time their male flowers shed pollen, protandrous (PA) individuals produced receptive female flowers after their male flowers shed pollen, and individuals that only produced female flowers were called female. To assess the overlap of female receptivity and pollen shed for both PG- and PA-morph types, we intensively monitored the flowering phenology of 28 of 59 total trees at 2–4-day intervals from May 17 to June 20 in 2002 employing the methods of Kimura et al. (2003). We measured tree height and diameter at breast height (dbh) for calculating basal area for each reproductive tree.
Sample collection and microsatellite genotyping
A sample of 405 seeds was collected from 11 mother trees (eight PG trees and three PA trees) on July in 2002 (Fig. 1). Sample size ranged from 28 to 53 seeds per mother tree. DNA was extracted from cotyledons of seeds (ca. 100 mg) or from leaves (50 mg) of all 57 trees that produced male flowers (two female trees were excluded), using a DNeasy Plant Mini Kit following the manufacturer’s protocol (QIAGEN). Out of 11 microsatellite loci used in this study (Table 1), 8 primer pairs (WGA001, 004, 007, 054, 072, 118, 142, and 256) were published in previous studies (Dangl et al. 2005; Woeste et al. 2002; Ross-Davis and Woeste 2008). The remaining three primer pairs (WGA015, 109, and 195) were newly developed (Table 2).
PCR amplifications were performed in 15-μL reaction volumes containing 10 ng genomic DNA as template, 1.5 μL of GeneAmp 10X PCR Gold buffer (Applied Biosystems), 1.5 μL of 2 mM each dNTP, 0.9 μL of 25 mM MgCl2, 0.3 μL of each 25 μM primer and 0.5 units of AmpliTaq Gold (Applied Biosystems). PCR amplifications were carried out for 10 min at 95 ºC, followed by 35 cycles of 20 s at 94 ºC and 30 s at 50 ºC, with a final 7-min incubation at 72 ºC using a GeneAmp PCR system 9700 (Applied Biosystems) or i-Cycler (Bio-Rad Laboratories, Inc). Fragment analysis was carried out using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems) or ABI PRISM 3100 avant Genetic Analyzer (Applied Biosystems), and each fragment size was compared with a Liz-labeled 500-bp standard using GeneScan program (Applied Biosystems) and ABI PRISM GenoTyper (Applied Biosystems).
Paternity analysis
The software CERVUS version 3.0 (Kalinowski et al. 2007) was employed to calculate number of alleles (N A), observed and expected heterozygosity (H O, H E), and non-paternity exclusion probability (N PE). Paternity exclusion probability (P E) was calculated as P E = 1 − N PE. Paternity of each seed was assigned preliminarily by the simple exclusion method, assuming all trees with male flowers (n = 57) were candidate pollen donors. In the initial analysis, we excluded miss-matched fathers using five loci (WGA 109, 118, 142, 195, and 256). If all 57 trees were excluded as pollen donors, we considered the seed to be the product of gene flow from outside the study area. In cases where one individual was selected by this exclusion procedure, we assigned it as a pollen parent of a seed. In case where more than one individual could have sired the seed (multiple matches), WGA 001, 004, 007, 015, 054, and 072 were used in the subsequent analysis, which proceeded as described earlier. If a seed had multiple potential male parents after exclusion with 11 microsatellite loci, we categorically assigned the most-likely pollen donor (Meagher 1986) with the highest LOD score (see Kalinowski et al. 2007).
For one mother tree (J04), the maternal genotype at WGA109 was 118/149, but 10 of 40 seeds from this parent appeared to have inherited a 138-bp allele. We evaluated the possibility of a sport (bud mutation) in this tree by collecting leaf samples from five branches and comparing their genotypes. We observed that leaves from four branches were consistent with previous data (118/149), but the leaves of the fifth branch were different (118/138). We concluded that the tree was a chimera of two genotypes. Subsequently, we regarded (J04) as two candidate pollen donors (J04a and J04b) for paternity analysis, and for the calculation of number of seeds sired by J04, we summed the number of those sired by J04a and J04b.
When the same tree was both mother and assigned as the father of the seed, we defined it as selfing. The selfing rate was calculated for each mother tree. For outcrossing within the study site, we compared sexual morphotype (PG or PA) of the mother tree with that of the assigned father. Mating events were categorically assigned as inter-morph mating (PG–PA) or intra-morph mating (PG–PG or PA–PA). Finally, we assigned four mating types: (1) selfing, (2) outcrossing by immigrant pollen dispersed from outside the quadrat, (3) inter-morph mating, and (4) intra-morph mating. For this study, we defined disassortative mating as inter-morph mating, whereas the assortative mating included selfing and intra-morph mating.
Data analysis
Using paternity analysis, we clarified the effects of mother morph type and the density of neighboring (30, 50, 100, and 200 m radius from each mother tree) inter-morph trees and intra-morph trees on the proportions of intra-morph mating. A generalized linear model (GLM) with a binomial error structure and a log link function was used for the analysis. In the GLM, the proportion of intra-morph mating was the response variable, while mother morph type, neighbourhood density of inter-morph trees and that of intra-morph trees were used as explanatory variables. For construction of the model, we supposed that mating mainly occur within the effective neighbourhood area. Then, four different neighbourhood areas with 30-, 50-, 100-, and 200-m radius were tested to determine the effective neighbourhood area. Finally, we selected the best model with the effective neighbourhood area based on Akaike’s information criterion (AIC). Statistical analysis was performed with R ver.2.15.0 (R Development Core Team 2012).
Modeling of pollen dispersal parameters
We estimated the immigration rate, selfing rate, and the average distance of pollen dispersal using the Bayesian mixed effect mating model (MEMM) approach (Klein et al. 2008, 2011). Genotype data at eight loci of 59 reproductive trees and 405 offspring and field data such as morph, basal area, and height of each reproductive tree were used for this estimation. The exponential power distribution was used for the dispersal kernel and Γ the classical gamma function. Similar to the existing Neighbourhood model (Burczyk et al. 2002), we assumed that the pollen pool composition depended not on the absolute fecundities but on relative fecundities. For the mating model and probabilities of offspring genotypes, the probability of fertilization of an ovule of a mother j was given as the probabilities s, m, and (1 − s − m), where (i) s was the probability pollen was from the mother tree (self-fertilization), (ii) m was the probability pollen was from an uncensused father tree from outside the study site (immigration), and (iii) the probability that pollen was from any censused father within the study site was (1 − s − m). The distribution of individual fecundity was assumed to be log-normal. The posterior distributions of (i) the variance of (log-) fecundity (σ2) to infer the ratio dobs/dep, (ii) the dispersal parameters (mean dispersal distance, scale parameter; δ, shape parameter; b) and the mating system parameters (s, m), and (iii) mean and standard deviation of individual fecundity (F k) of each reproductive tree were calculated by MCMC algorithm (Klein et al. 2008).
Results
Morph ratio and phenological overlap
Flowering phenology was observed for all 59 reproductive trees during May and June of 2002 and 2003. There were 41 PG trees, 16 PA trees, and two female trees in the quadrat. The ratio of PG versus PA was significantly different from 1:1 (χ2 = 10.96, df = 1, p < 0.001).
Figure 2 illustrates the reciprocity and synchronization of flowering times of PG trees (n = 21) and PA trees (n = 7). Peak pollen shed in PG trees was 3 days later than peak receptivity of female flowers. Similarly, the peak of female receptivity in PA trees was about 4 days after peak pollen shed. Within individuals, the flowering period of female and male flowers overlapped for 1 or 2 days for 21 (16 PG and 5 PA trees) out of 28 trees, so most trees appeared to have an opportunity to self-pollinate. Bloom overlap within morphs was about 13/20 days for PG trees and about 10/20 days for PA trees, so same-morph males had about half as much time to mate with same-morph females as opposite-morph males.
The distance distribution of the intra- and the inter-morph trees from PG and PA trees indicated that PG trees clearly suffered from an excess of intra-morph mates compared with PA trees (Fig. 3).
Paternity analysis
The 11 microsatellite markers we used for genotyping were highly variable, having 5–25 alleles per locus, with an average of 13.8. The observed and expected heterozygosities ranged from 0.348 to 0.793 and from 0.371 to 0.893, respectively (Table 1). None of the loci showed significant deviation from Hardy–Weinberg equilibrium, so mating in the population can be considered random. The total exclusion probability (P E) calculated for the first 5, 8, and 11 loci were 0.9969, 0.9990, and 0.9999 respectively, indicating that the microsatellites had a high level of power to distinguish paternity.
Of 405 seeds genotyped, the pollen donor of 194 seeds (47.9 %) was not assigned to any candidates within the quadrat. We considered these seeds to be the products of gene flow from outside the study area. Selfing was detected in 56 seeds (13.8 % of the total). Paternity of the remaining 155 seeds that derived from outcrossing was assigned to a single tree within the quadrat for each offspring. Intra-morph and inter-morph mating was detected in 21 seeds (5.2 %) and 134 seeds (33.1 %), respectively.
For PG trees, the proportion of seeds derived from mating with trees outside the study area (immigrant pollen) averaged 55.3 % and ranged from 28.1 to 75 % (light grey wedges, Fig. 1). For PA trees, the proportion of seeds derived from immigrant pollen averaged 28.9 % and ranged from 12.5 to 55.9 %. The selfing rate (black wedge, Fig. 1) for PG trees averaged 14.8 % and ranged from 0 to 37.7 %; for PA trees, the selfing rate averaged 11.4 % and ranged from 5.0 to 26.5 %. Intra-morph mating as a proportion of total outcrossing within the quadrat averaged 23.0 % (20/87) for PG trees and ranged from 0 to 73.3 %, whereas it averaged 1.5 % (1/68) for PA trees, and ranged from 0 to 3.4 % (dark grey wedges, Fig. 1).
We used AIC values to choose the most appropriate neighbourhood area; AIC values for neighbourhoods with 200-, 100-, 50-, and 30-m radius were 52.3, 37.1, 32.0, and 50.4, respectively. Thus, we selected the 50-m radius as the best neighbourhood model. The proportion of intra-morph mating for PG-mothers was significantly higher than that of PA-mothers. Density of neighboring inter-morph and intra-morph trees had a significant effect on frequency of intra-morph mating (Table 3).
Modeling of pollen dispersal parameters
Mean, median, and 95 % confidence interval of pollen dispersal distance (δ), immigration rate (m), and the selfing rate (s) for PG- and PA-mother trees were summarized in Table 4. The posterior mean values of δ for PG- and PA-mother trees were 392 and 233 m, respectively. Thus, PG-mother trees had a larger posterior mean than PA-mother ones. The confidence interval of δ was large, especially for PG-mother trees. The values of immigration and selfing rates were similar to those obtained from paternity analysis.
Individual fecundity
The posterior mean fecundity (F k) and its standard deviation for each reproductive tree for both PG- and PA-mothers are shown in Fig. 4. Some individual PG- and PA-mothers showed markedly higher individual inter-morph fecundity (F k > 3) than other trees in the population, i.e., PG-mother trees J16 and J40 and PA-mother trees J22, J33, J34, and J39. A few intra-morph trees (e.g., J03 and J33) also exhibited high individual intra-morph fecundity (F k > 3) but these were observed PG-mother (majority-morph) trees only.
Discussion
Mating system
The mating system of walnuts, the genus Juglans, is characterized by a protogynous–protandrous dimorphism which is a form of heterodichogamy. A genetic model of this system predicts a stable polymorphism with both mating types in equal proportion (Gleeson 1982). Nevertheless, the morph ratio of our study population was significantly biased from 1:1 there were more than twice as many PG trees as PA trees. This deviation from the expected ratio could be caused by demographic stochasticity (Kery et al. 2003) in a small population at local scale. We showed that majority-morph (PG) trees had significantly more intra-morph mates than PA trees in the low-density and morph-ratio-biased population we studied (Fig. 3).
Recently, Bai et al. (2007) showed that selfing was rare (for PG and PA trees it was 3.8 and 1.4 %, respectively) in a high-density morph-ratio-unbiased population of the heterodichogamous tree J. mandshurica, which is a close congener of J. ailantifolia. This indicates that heterodichogamy can effectively reduce selfing and promote outcrossing. In our low-density and morph-ratio-biased population of J. ailantifolia, however, the selfing rates of PG and PA trees derived from both paternity analysis and MEMM were 15 and 11 %, respectively (Fig. 1; Table 4). Although little difference was observed between PG and PA trees, these values were remarkably higher than those observed by Bai et al. (2007). Furthermore, despite our observation that majority-morph (PG) trees had significantly fewer potential of inter-morph mates at close range than minority-morph trees, the selfing rate of PG- and PA-mother trees was similar. The high rate of selfing (e.g., in J13 and J31; Fig. 1) we observed did not appear to be a mechanism for assuring reproduction in response to the low-density and morph-ratio-bias of the population.
In this study, of the seeds with paternities assigned to trees within the quadrat, the proportion of intra-morph mating was higher in PG trees (23.0 %) than in PA trees (1.5 %). Morph-specific differences in intra-morph mating were also reported in a morph-biased (PG/PA-ratio biased) population of the heterodichogamous tree, Acer opalus (Gleiser et al. 2008b). They also indicated that 21.2 % of seeds derived from majority-morph (PG) trees were produced by intra-morph mating, whereas no intra-morph mating was observed in seeds derived from PA trees. By contrast, intra-morph mating in PG trees (4.3 %) was almost identical to that in PA trees (7.1 %) in J. mandshurica in a morph-ratio-unbiased population (Bai et al. 2007). These observations strongly suggest that in morph-ratio-biased populations, the proportion of intra-morph mating for majority-morph trees is significantly higher than that for minority-morph trees. The simplest explanation for these results is the difference in availability of mating partners (inter-morph trees) in the neighbourhood. In this study, we found that the density of neighboring inter-morph trees and intra-morph trees (radius = 50 m) significantly affected the proportion of intra-morph matings (Table 3). The neighbourhood we chose (50 m) was reasonable because distance significantly affects male fecundity, and a 50-m neighbourhood is comparable to values from other species (Burczyk et al. 2002; Goto et al. 2006).
In the J. ailantifolia population studied, the intra-morph male and female bloom period overlapped over 10 days within the intra-morph type (Fig. 2), and 75 % of the trees showed 1 or 2 days overlap within tree. By contrast, in a high-density and morph-ratio-unbiased population of J. mandshurica, female and male flowers were almost entirely separated temporally within same-morph (Bai et al. 2006). Assortative mating, selfing, and intra-morph mating are expected to increase with increased male and female bloom overlap within mother trees or among trees of the same morphotype. Therefore, a relatively lengthy bloom overlap may have affected the extent of assortative mating in this study.
Pollen dispersal
The mean distance of pollen dispersal (δ) was 392 m for PG-mother trees, and 233 m for PA-mother trees. The immigration rates were approximately 58 % and 29 % for PG- and PA-mother trees, respectively. These findings indicate that long-distance dispersal is common in this population (Fig. 1; Table 4). Bai et al. (2007) showed that pollen dispersal in J. mandshurica was predominantly short-distance, and the distribution of distance between mates was similar between both morphs in a high-density and morph-ratio-unbiased population. Local density of reproductive trees affects the patterns of pollen dispersal in forest trees. Long-distance pollen dispersal tends to be promoted in a fragmented or a low-density population (Stacy et al. 1996; White et al. 2002; Bacles and Ennos 2008). Our study revealed that long-distance pollen flow can play an important role in the reproduction of a morph-biased populations of Juglans ailanthifolia.
The pollen dispersal (δ) was higher in PG-mother trees than PA-mother trees (Table 4). Confidence intervals, however, were quite large, especially for PG-mother trees. Furthermore, PG-mother trees used immigrant pollen at a rate significantly higher than PA-mother trees. The mean and variance of the distance distribution for immigrant pollen were unknown, although based on the composition of the surrounding forest, immigrant pollen was probably the result of long-distance dispersal. Given these facts, we could not determine whether the pollen dispersal distance of PG-mother trees was longer than that of PA-mother trees.
Individual fecundity
Several PG- and PA-mothers exhibited markedly higher inter-morph fecundity (F k > 3) than other trees in the population Fig. 4). This finding revealed that assortative mating effectively occurred in a morph-biased population of Juglans ailantifolia. Only among PG (majority-morph) trees, however, did we observe individuals with unusually high individual intra-morph fecundity. Thus, we suggest that intra-morph mating is favored when inter-morph trees are not available within the neighbourhood.
Conclusions
In this study, we found that a heterodichogamous tree in a deforested (low-density) population deviated significantly from the expected 1:1 morph-ratio. Nevertheless, as a result of paternity and statistical analysis, we found that PG- and PA-mother trees did not differ for selfing rate or pollen dispersal distance. Long-distance dispersal of pollen onto mothers of both morphotypes probably plays an important role in maintaining the studied population. The proportion of intra-morph mating was higher in the majority-morph mother trees than in the minority-morph mother trees. Furthermore, only among PG (majority-morph) trees did we observe individuals with unusually high individual intra-morph fecundity. These findings suggested that intra-morph mating may occur when majority-morph mothers suffer a deficiency of local mating partners (inter-morph trees).
References
Bacles CFE, Ennos RA (2008) Paternity analysis of pollen-mediated gene flow for Fraxinus excelsior L. in a chronically fragmented landscape. Heredity 101:368–380
Bai WN, Zeng YF, Liao WJ, Zhang DY (2006) Flowering phenology and wind pollination efficacy of heterodichogamous Juglans mandshurica (Juglandaceae). Ann Bot 98:397–402
Bai WN, Zeng YF, Zhang DY (2007) Mating patterns and pollen dispersal in a heterodichogamous tree, Juglans mandshurica (Juglandaceae). New Phyt 176:699–707
Burczyk J, Adams WT, Moran GF, Griffin AR (2002) Complex patterns of mating revealed in a Eucalyptus regnans seed orchard using allozyme markers and the neighbourhood model. Mol Ecol 11:2379–2391
Dangl G, Woeste K, Aradhya M, Koehmstedt A, Simon C, Potter D, Leslie C, McGranahan G (2005) Characterization of 14 microsatellite markers for genetic analysis and cultivar identification of walnut. J Am Soc Hort Sci 130:348–358
Eckert CG, Kalisz S, Geber MA, Sargent R, Elle E, Cheptou PO, Goodwillie C, Johnston MO, Kelly JK, Moeller DA, Porcher E, Ree RH, Vallejo-Marín M, Winn AA (2010) Plant mating systems in a changing world. Trends Ecol Evol 24:35–43
Gleeson S (1982) Heterodichogamy in walnuts: inheritance and stable ratios. Evolution 36:892–902
Gleiser G, Segarra-Moragues J, Pannell J, Verdu M (2008a) Siring success and paternal effects in heterodichogamous Acer opalus. Ann Bot 101:1017–1026
Gleiser G, Verd′u M, Segarra-Moragues J, Gonz′alez-Mart′ınez S, Pannell J, Shykoff J (2008b) Disassortative mating, sexual specialization, and the evolution of gender dimorphism in heterodichogamous Acer opalus. Evolution 62:1676–1688
Goto S, Shimatani K, Yoshimaru H, Takahashi Y (2006) Fat-tailed gene flow in the dioecious canopy tree species Fraxinus mandshurica var. japonica revealed by microsatellites. Mol Ecol 15:2985–2996
Ishihama F, Nakano C, Ueno S, Ajima M, Tsumura Y, Washitani I (2003) Seed set and gene flow patterns in an experimental population of an endangered heterostylous herb with controlled local opposite-morph density. Funct Ecol 17:680–689
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer rogram cervus accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kery M, Matthies D, Schmid B (2003) Demographic stochasticity in population fragments of the declining distylous perennial Primula veris (Primulaceae). Basic Appl Ecol 4:197–206
Kikuchi S, Shibata M, Tanaka H, Yoshimaru H, Niiyama K (2009) Analysis of the disassortative mating pattern in a heterodichogamous plant, Acer mono maxim. using microsatellite markers. Plant Ecol 204:43–54
Kimura M, Seiwa K, Suyama Y, Ueno N (2003) Flowering system of heterodichogamous Juglans ailanthifolia. Plant Species Biol 18:75–84
Klein EK, Desassis N, Oddou-Muratorio S (2008) Pollen flow in the wildservice tree, Sorbus torminalis (L.) Crantz. IV. Whole interindividual variance of male fecundity estimated jointly with the dispersal kernel. Mol Ecol 17:3323–3336
Klein EK, Carpentier FH, Oddou-Muratorio S (2011) Estimating the variance of male fecundity from genotypes of progeny arrays: evaluation of the Bayesian forward approach. Methods Ecol Evol 2:349–361
Matsui M, Goto S, Shibano S, Kimura M, Suyama Y (2002) Distribution and regeneration of Juglans ailanthifolia in natural forests of central Hokkaido. Trans Meeting Hokkaido Br Jpn J For Soc 50:68–70 (in Japanese)
Meagher TR (1986) Analysis of paternity within a natural-population of Chamaelirium-luteum.1. identification of most-likely male parents. Am Nat 128:199–215
Renner SS (2001) How common is heterodichogamy? Trends Ecol Evol 16:595–597
Ross-Davis A, Woeste KE (2008) Microsatellite markers for Juglans cinerea L. and their utility in other Juglandaceae species. Conserv Genet 9:465–469
Stacy E, Hamrick J, Nason J, Hubbell S, Foster R, Condit R (1996) Pollen dispersal in low-density populations of three neotropical tree species. Am Nat 148:275–298
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org/
Thompson TE, Romberg LD (1985) Inheritance of heterodichogamy in pecan. J Hered 76:456–458
Watanabe S, Sasaki S (1994) The silvicultural management-system in temperate and boreal forests: a case-history of the Hokkaido Tokyo-University Forest. Can J For Res 24:1176–1185
White GM, Boshier DH, Powell W (2002) Increased pollen flow counteracts fragmentation in a tropical dry forest: an example from Swietenia humilis zuccarini. Proc Natl Acad Sci USA 99:2038–2042
Woeste K, Burns R, Rhodes O, Michler C (2002) Thirty polymorphic nuclear microsatellite loci from black walnut. J Hered 93:58–60
Acknowledgments
We thank S. Shibano, Y. Takahashi, N. Kimura, K. Uchiyama, K. Okamura, and the technical staff of University Forest in Hokkaido, the University of Tokyo for help with field measurement and Dr Y. Nakai for use of his laboratory facility. We greatly appreciate Dr EK Klein’s support in the application of the mixed mating model based on Bayesian approach. We also thank Dr H. Iwata and Dr M. Tomita for his helpful comments on modelling of pollen dispersal and Dr N. Miura for English editing. This research was supported by grants from the Japan Society for the Promotion of Science (No. 12460064) to KS and (No. 22380080) to SG, the River Environment Fund in charge of the Foundation of River and Watershed Environment Management (No. 11-4-5) to KS, the Sasakawa Scientific Research Grant from The Japan Science Society to MK and Grant-in-Aid for JSPS Fellows to MK. The use of trade names is for the information and convenience of the reader and does not imply official endorsement or approval by the United States Department of Agriculture or the Forest Service of any product to the exclusion of others that may be suitable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimura, M.K., Goto, S., Suyama, Y. et al. Morph-specific mating patterns in a low-density population of a heterodichogamous tree, Juglans ailantifolia . Plant Ecol 213, 1477–1487 (2012). https://doi.org/10.1007/s11258-012-0105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-012-0105-6