Abstract
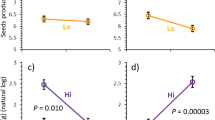
The activation of dormant meristems following apical damage is an important mechanism for tolerance of herbivore damage, but its impact could vary with resource availability. Here, we examined central predictions of the limiting resource model (LRM), according to which high resource availability can support damage tolerance in plants with deterministic apical dominance, but will have limited or no effect in plants that are induced to increase branching by increased resource availability regardless of damage. We examined these predictions by studying the branching patterns of Medicago truncatula plants in response to both light and water availabilities and their effects on tolerance of apical damage. We used plants from environments that were predicted to select for different levels of apical dominance. Intact plants from the more productive and competitive population exhibited strong apical dominance and refrained from branching even under full light, whereas plants from the less productive and sparser population exhibited greater plasticity in apical dominance and readily branched under high water and light. In accordance with the LRM, these differences translated into differential responsiveness to apical damage: given abundant water, apical damage induced the activation of lateral meristems and increased pod and seed production in plants from the more productive environment, but not in plants from the less productive environment. These results suggest an adaptive association between deterministic inhibition of lateral meristems and compensatory ability, which supports the hypothesis that greater compensatory responsiveness to apical damage could be a derivative of adaptation to other environmental stresses, such as light competition.


Similar content being viewed by others
References
Aarssen LW (1995) Hypotheses for the evolution of apical dominance in plants: implications for the interpretation of overcompensation. Oikos 74:149–156
Aarssen LW, Irwin DL (1991) What selection: herbivory or competition. Oikos 60:261–262
Alpert P, Simms EL (2002) The relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evol Ecol 16:285–297
Amir S, Cohen D (1990) Optimal reproductive efforts and the timing of reproduction of annual plants in randomly varying environments. J Theor Biol 147:17–42
Aronson JA, Kigel J, Shmida A (1990) Comparative plant sizes and reproductive strategies in desert and Mediterranean populations of ephemeral plants. Isr J Bot 39:413–430
Banta JA, Stevens MHH, Pigliucci M (2010) A comprehensive test of the ‘limiting resources’ framework applied to plant tolerance to apical meristem damage. Oikos 119:359–369
Belsky AJ (1986) Does herbivory benefit plants: a review of the evidence. Am Nat 127:870–892
Bigger DS, Marvier MA (1998) How different would a world without herbivory be? A search for generality in ecology. Integr Biol 1:60–67
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. Advan Genet 13:115–155
Bruins HJ (1990) The impact of man and climate on the central Negev and northeastern Sinai Deserts during the late Holocene. In: Bottenma SS, Entjes-Nielborg G, van Zeist W (eds) Man’s role in the shaping of the Eastern Mediterranean landscape. Balkema, Rotterdam, pp 87–99
Cohen D (1971) Maximizing final yield when growth is limited by time or by limiting resources. J Theor Biol 33:299–307
Crawley MJ (1983) Herbivory: The dynamics of animal–plant interactions. Blackwell, Oxford
Ferraro DO, Oesterheld M (2002) Effect of defoliation on grass growth. A quantitative review. Oikos 98:125–133
Gambash S, Kochba M, Avnimelech Y (1990) Studies on slow-release fertilizers: II. A method for evaluation of nutrient release rate from slow-releasing fertilizers. Soil Sci 150:446–450
Goldshleger N, Ben-Dor E, Benyamini Y, Agassi M (2004) Soil reflectance as a tool for assessing physical crust arrangement of four typical soils in Israel. Soil Sci 169:677–687
Grime JP (1979) Plant strategies and vegetation processes. John Wiley & Sons, Chichester
Hawkes CV, Sullivan JJ (2001) The impact of herbivory on plants in different resource conditions: a meta-analysis. Ecology 82:2045–2058
Hilbert DW, Swift DM, Detling JK, Dyer MI (1981) Relative growth-rates and the grazing optimization hypothesis. Oecologia 51:14–18
Huhta AP, Lennartsson T, Tuomi J, Rautio P, Laine K (2000) Tolerance of Gentianella campestris in relation to damage intensity: an interplay between apical dominance and herbivory. Evol Ecol 14:373–392
Johnson EA, Miyanishi K (2007) Plant disturbance ecology: the process and the response. Elsevier, Burlington
Juenger T, Bergelson J (2000) The evolution of compensation to herbivory in scarlet gilia, Ipomopsis aggregata: herbivore-imposed natural selection and the quantitative genetics of tolerance. Evolution 54:764–777
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
King D, Roughgarden J (1982) Graded allocation between vegetative and reproductive growth for annual plants in growing seasons of random length. Theor Popul Biol 22:1–16
Kirk RE (1995) Experimental design: procedures for the behavioral sciences. Brooks/Cole, Pacific Grove, CA
Lesins KA, Lesins I (1979) Genus medicago leguminosae. Dr. W. Junk, The Hague
Limami AM, Ricoult C, Planchet E, et al (2007) Response of Medicago truncatula to abiotic stress. The Medicago truncatula handbook. http://www.noble.org/MedicagoHandbook/. Accessed 11 April 2010
Maschinski J, Whitham TG (1989) The continuum of plant-responses to herbivory: the influence of plant-association, nutrient availability, and timing. Am Nat 134:1–19
McConnaughay KDM, Coleman JS (1999) Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology 80:2581–2593
Moreau D (2006) Morphology, development and plant architecture of M. truncatula. The Medicago truncatula handbook. http://www.noble.org/MedicagoHandbook/. Accessed 1 Mar 2010
Novoplansky A (1996) Developmental responses of individual Onobrychis plants to spatial heterogeneity. Vegetatio 127:31–39
Novoplansky A (2002) Developmental plasticity in plants: implications of non-cognitive behavior. Evol Ecol 3:177–188
Novoplansky A (2009) Picking battles wisely: plant behaviour under competition. Plant Cell Environ 32:726–741
Novoplansky A, Cohen D, Sachs T (1994) Responses of an annual plant to temporal changes in light environment: an interplay between plasticity and determination. Oikos 69:437–446
Núñez-Farfán J, Fornoni J, Valverde PL (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38:541–566
Perevolotsky A, Seligman NG (1998) Role of grazing in Mediterranean rangeland ecosystems: inversion of a paradigm. Bioscience 48:1007–1017
Petrů M, Tielbörger K, Belkin R, Sternberg M, Jeltsch F (2006) Life history variation in an annual plant under two opposing environmental constraints along an aridity gradient. Ecography 29:66–74
Pigliucci M (1998) Developmental phenotypic plasticity: where internal programming meets the external environment. Curr Opin Plant Biol 1:87–91
Polley HW, Detling JK (1988) Herbivory tolerance of Agropyron smithii populations with different grazing histories. Oecologia 77:261–267
Rautio P, Huhta AP, Piippo S et al (2005) Overcompensation and adaptive plasticity of apical dominance in Erysimum strictum (Brassicaceae) in response to simulated browsing and resource availability. Oikos 111:179–191
Rolston MP (1978) Water impermeable seed dormancy. Bot Rev 44:365–396
Scheiner SM (2001) MANOVA: multiple response variables and multispecies interactions. In: Scheine SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman & Hall, New York, pp 99–115
Schmitt J (1997) Is photomorphogenic shade avoidance adaptive? Perspectives from population biology. Plant Cell Environ 20:826–830
Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Ann Rev Ecol Syst 31:565–595
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Sultan SE (2010) Plant developmental responses to the environment: eco-devo insights. Curr Opin Plant Biol 13:96–101
Tiffin P (2000) Mechanisms of tolerance to herbivore damage: what do we know? Evol Ecol 14:523–536
Trumble JT, Kolodnyhirsch DM, Ting IP (1993) Plant compensation for arthropod herbivory. Annu Rev Entomo 38:93–119
Weijschede J, Martinkova J, de Kroon H, Huber H (2008) Effects of cell number and cell size on petiole length variation in a stoloniferous herb. Am J Bot 95:41–49
Wienig C (2000) Plasticity versus canalization: population differences in the timing of shade-avoidance responses. Evolution 54:441–451
Wise MJ, Abrahamson WG (2005) Beyond the compensatory continuum: environmental resource levels and plant tolerance of herbivory. Oikos 109:417–428
Wise MJ, Abrahamson WG (2007) Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. Am Nat 169:443–454
Wise MJ, Abrahamson WG (2008) Applying the limiting resource model to plant tolerance of apical meristem damage. Am Nat 172:635–647
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, NJ
Zohary M (1987) Flora Palaestina, vol 2. Jerusalem, Israel Academy of Sciences and Humanities
Acknowledgments
We thank Omer Falik, Hagai Shemesh and Itamar Giladi for the stimulating discussions, Eliah Malka and Tali Brunner for helpful comments on early versions of the manuscript and Tania Acuña and Efrat Elimelech for the dedicated technical help. The study was supported in part by a research grant from the Israel Science Foundation to A.N. This is publication no. 732 of the Mitrani Department of Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gruntman, M., Shirata, C. & Novoplansky, A. Plasticity in apical dominance and damage tolerance under variable resource availability in Medicago truncatula . Plant Ecol 212, 1537–1548 (2011). https://doi.org/10.1007/s11258-011-9929-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-011-9929-8