Abstract
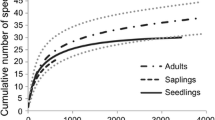
Detailed information on 38 species and 26 environmental variables was recorded from a network of 86 permanent plots across a geographical range of 10 km, in order to determine the patterns of floristic composition in Quercus-dominated forests; to elucidate environmental differentiation in such forests; and to determine whether species are partitioning their environment. To examine likely patterns of floristic composition, a data matrix expressed as relative volume + relative density was used to run non-metric multidimensional scaling. Canonical correspondence analysis extracted the environmental variation that best correlates with the observed patterns of floristic composition. Our results indicate that congeneric Quercus individuals represent the largest proportion of the species pool in the study plots. They coexist with other species having similar ecological requirements in at least three distinct floristic groups. Examination of the two largest groups and their species compositions reveals that one floristic gradient runs across the most xeric zone of the study area, and the second major floristic gradient runs across a mesic zone. The most important environmental variable explaining the observed patterns of floristic composition is altitude, although partial canonical correspondence analysis suggests that micro-habitat heterogeneity (catena position and canopy maturity) was most significant.




Similar content being viewed by others
References
Almeida AM, Prado PI, Lewinsohn T (2004) Geographical distribution of Eupatorieae (Asteraceae) in South-eastern and South Brazilian mountain ranges. Plant Ecol 174:163–181
Beatty SW (1984) Influence of microtopography and canopy species on spatial patterns of forest understory plants. Ecology 65:1406–1419
Bell G, Lechowicz MJ, Waterway MJ (2000) Environmental heterogeneity and species diversity of forest sedges. J Ecol 88:67–87
Bowman DMJS, Minchin PR (1987) Environmental relationships of woody vegetation patterns in the Australian Monsoon Tropics. Aust J Bot 35:151–169
Brais S, Camiré C, Bergeron Y, Paré D (1995) Changes in nutrient availability and forest floor characteristics in relation to stand age and forest composition in the southern part of the boreal forest of northwestern Quebec. For Ecol Manag 76:181–189
Collins BS, Battaglia LL (2002) Microenvironmental heterogeneity and Quercus michauxxi regeneration in experimental gaps. For Ecol Manag 155:279–290
Cuevas-Guzmán R, Benz BF, Jardel PE (1997) Sierra de Manantlán. In: Heywood DS, Herrera-MacBryde O, Villalobos J, Hamilton AC (eds) Centres of plant diversity. World Conservation Union-World Wildlife Fund, Washington, pp 158–161
Dalberg Poulsen A, Tuomisto H, Blaslev H (2006) Edaphic and floristic variation within a 1-ha plot of lowland Amazonian rain forest. Biotropica 38:468–478
Fekedulegn D, Hicks RR Jr, Colbert JJ (2003) Influence of topographic aspect, precipitation and drought on radial growth of four major tree species in an Appalachian watershed. For Ecol Manag 177:409–425
Forcier LK (1975) Reproductive strategies and the co-occurrence of climax tree species. Science 189:808–810
Gerhardt F, Foster DR (2002) Physiographical and historical effects on forest vegetation in central New England, USA. J Biogeogr 29:1421–1437
Gilbert B, Lechowicz M (2004) Neutrality, niches, and dispersal in a temperate forest understory. Proc Natl Acad Sci USA 101:7651–7656
Gotelli N, McCabe D (2002) Species co-occurrence: a meta-analysis of J.M. Diamond’s assembly rules model. Ecology 83:2091–2096
Guo Q (1998) Microhabitat differentiation in Chihuahuan desert plant communities. Plant Ecol 139:71–80
Hardy O, Sonké B (2004) Spatial pattern analysis of tree species distribution in a tropical rain forest of Cameroon: assessing the role of limited dispersal and niche differentiation. For Ecol Manag 197:191–202
Hausdorf B, Henning C (2007) Null model test of clustering of species, negative co-occurrence patterns and nestedness in meta-communities. Oikos 116:818–828
Heikkinen RK, Birks HJB, Kalliola R (1998) A numerical analysis of the mesoscale distribution patterns of vascular plants in the subartic Kevo Nature Reserve, northern Finland. J Biog 25:123–146
Hemp A (2006) Continuum or zonation? Altitudinal gradients in the forest vegetation of Mt. Kilimanjaro. Plant Ecol 184:27–42
Hirayama K, Sakimoto M (2003) Spatial distribution of canopy and subcanopy species along a sloping topography in a cool-temperate conifer-hardwood forest in the snowy region of Japan. Ecol Res 18:443–454
Hofer U, Bersier L-F, Borcard D (2004) Relating niche and spatial overlap at the community level. Oikos 106:366–376
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Husch B, Miller CI, Beers TW (1982) Forest mensuration. Wiley, New York
Jones MM, Tuomisto H, Clark DB, Olivas P (2006) Effects of mesoscale environmental heterogeneity and dispersal limitation on floristic variation in rain forest ferns. J Ecol 94:181–195
Kappelle M (2006) Structure and composition of Costa Rican montane oak forests. In: Kappelle M (ed) Ecology and conservation of neotropical montane oak Forests. Springer-Verlag, Berlin, pp 127–139
Kappelle M, Cleef AM, Chavarri A (1992) Phytogeography of Talamancan montane Quercus forests, Costa Rica. J Biogeogr 19:299–315
Kariuki M, Rolfe M, Smith RGB, Vanclay JK, Kooyman RM (2006) Diameter growth performance varies with species functional-group and habitat characteristics in subtropical rainforests. For Ecol Manag 225:1–14
Kelly CK, Bowler M, Pybus O, Harvey P (2008) Phylogeny, niches, and relative abundance in natural communities. Ecology 89:962–970
Kruskal JB (1964) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:115–129
Lazcano SC (1978) Las cavernas de Cerro Grande, estados de Jalisco y Colima. Laboratorio Natural Las Joyas. Universidad de Guadalajara, Guadalajara, Jalisco
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science, Amsterdam
Liebhold A, Koening WD, Bjornstad ON (2004) Spatial synchrony in population dynamics. Ann Rev Ecol Syst 35:467–490
Loehle C (2000) Strategy space and the disturbance spectrum: a life-history model for tree species coexistence. Am Nat 156:14–33
Luna-Vega I, Alcántara-Ayala O, Ruíz-Jiménez CA, Contreras-Medina R (2006a) Composition and structure of humid montane oak forests at different sites in Central and Eastern Mexico. In: Kappelle M (ed) Ecology and conservation of neotropical montane Oak forests. Springer-Verlag, Berlin, pp 102–112
Luna-Vega I, Alcántara Ayala O, Contreras Medina R, Ponce Vargas A (2006b) Biogeography, current knowledge and conservation of threatened vascular plants characteristic of Mexican temperate forests. Biodiversity Conserv 15:3773–3799
Marshall CJ, Liebherr JK (2000) Cladistic biogeography of the Mexican transition zone. J Biogeogr 27:203–216
McCune B, Mefford MJ (1999) PC-ORD. Multivariate analysis of ecological data, version 4. MjM Software Design, Gleneden Beach
McNab WH (1993) A topographic index to quantify the effect of mesoscale landform on site productivity. Can J For Res 23:1100–1107
Meave JA, Rincón A, Romero-Romero MA (2006) Oak forests of the hyper-humid region of La Chinantla, Northern Oaxaca Range, Mexico. In: Kappelle M (ed) Ecology and conservation of neotropical montane oak forests. Springer-Verlag, Berlin, pp 113–125
Mohler CL (1990) Co-occurrence of oak subgenera: implications for niche differentiation. Bull Torrey Bot Club 117:247
Nakashizauka T (2001) Species coexistence in temperate, mixed deciduous forests. Trends Ecol Evol 16:205–210
Økland RH (1996) Are ordination and constrained ordination alternative or complementary strategies in general ecological studies? J Veg Sci 7:289–292
Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, O’Hara RG, Simpson GL, Solymos P, Stevens H, Wagner H (2010) vegan: community ecology package. R package, version 1.18-2/r1135. http://R-Forge.R-project.org/projects/vegan/
Olvera-Vargas M (2006) Spatio-temporal dynamics of Neotropical high-altitude mixed-oak forest in Western Mexico. Doctoral Thesis, University of Oxford
Olvera-Vargas M, Moreno Gómez S, Figueroa-Rangel BL (1996) Sitios permanentes de investigación Silvícola: Manual para su establecimiento. Universidad de Guadalajara, Guadalajara
Olvera-Vargas M, Figueroa-Rangel BL, Moreno Gómez S, Solís-Magallanes A (1998) Resultados preliminares de la fenología de cuatro especies de encino (Quercus) en Cerro Grande, Reserva de la Biosfera Sierra de Manantlán. Biotam 9:7–18
Ozinga WA, Schaminée HJ, Bekker RM, Bonn S, Poschlod P, Tackenberg O, Bakker J, van Groenendal JM (2005) Predictability of plant species composition from environmental conditions is constrained by dispersal limitation. Oikos 108:555–561
Padilla-Velarde E, Cuevas-Guzmán R, Ibarra-Manríquez G, Moreno Gómez S (2006) Ríqueza y biogeografía de la flora arbórea de Colima. Revista Mexicana de Biodiversidad 77
Park AD (2001) Environmental influences on post-harvest natural regeneration in Mexican pine-oak forests. For Ecol Manag 144:213–228
Park A (2003) Spatial segregation of pines and oaks under different fire regimes in the Sierra Madre Occidental. Plant Ecol 169:1–20
Poorter L, Arets E (2003) Light environment and tree strategies in a Bolivian tropical moist forest: an evaluation of the light partitioning hypothesis. Plant Ecol 166:295–306
Ramírez-Marcial N, Ochoa-Gaona S, González-Espinoza M, Quintana-Ascencio PF (1998) Análisis florístico y sucesional en la Estación Biológica Cerro Huitepec, Chiapas, México. Acta Bot Mex 44:59–85
Ramírez-Marcial N, Camacho-Cruz A, González-Espinoza M, López-Barrera F (2006) Establishment, survival and growth of tree seedlings under successional montane oak forests in Chiapas Mexico. In: Kappelle M (ed) Ecology and conservation of neotropical montane oak forests. Springer-Verlag, Berlin, pp 177–189
Rzedowski J (1978) La vegetación de México. Editorial Limusa, México City
Sakai A, Ohsawa M (1993) Vegetation pattern and microtopography on a landslide scar of Mt Kiyosumi, central Japan. Ecol Res 8:47–56
Slik JWF, Poulsen AD, Ashton PS, Cannon CH, Eichhorn KAO, Kartawinata K, Lanniari I, Nagamasu H, Nakagawa M, van Nieuwstadt MGL, Payne J, Purwaningsih A, Sardan K, Sidiyasa K, Verburg RW, Webb CO, Wilkie P (2003) A floristic analysis of the lowland dipterocarp forests of Borneo. J Biogeogr 30:1517–1531
Svenning J-C (2001) Microhabitat specialization in a species-rich palm community in Amazonian Ecuador. J Ecol 87:55–65
Svenning J-C, Kinner DA, Stallard RF, Engelbrecht BMJ, Wright SJ (2004) Ecological determinism in plant community structure across a tropical forest landscape. Ecology 85:2526–2538
ter Braak CJF, Smilauer P (1998) CANOCO reference manual and user’s guide to Canoco for Windows: software for canonical community ordination, version 4. Centre for Biometry Wageningen, CPRO-DLO, Wageningen
Tokeshi M (1999) Species coexistence: ecological and evolutionary perspectives. Blackwell Science, Oxford
Tuomisto H, Ruokolainen K, Aguilar M, Sarmiento A (2003a) Floristic patterns along a 43-km long transect in an Amazonian rain forest. J Ecol 91:743–756
Tuomisto H, Ruokolainen K, Yli-Halla M (2003b) Dispersal, environment, and floristic variation of western Amazonian forests. Science 299:241–244
Valencia S (2004) Diversidad del género Quercus (Fagaceae) en México. Bol Soc Bot Mex 075:33–53
Vázquez-García JA, Cuevas-Guzmán R, Cochrane TS, Iltis HH, Santana-Michel FJ, Guzmán-Hernández L (1995) Flora de Manantlán. Botanical Research Institute of Texas, Inc, Fort Worth
Vormisto J, Svenning J-C, Hall P, Balslev H (2004) Diversity and dominance in palm (Arecaceae) communities in terra firme forests in the western Amazon basin. J Ecol 92:577–588
Acknowledgements
We are greatly indebted to Oscar Sánchez Jiménez, Jose María Michel Fuentes and Abel Ceja Gutiérrez for their valuable assistance during the fieldwork. To Saúl Moreno Gómez, for his great help during the early plot establishment. We thank Professor Frans Bongers, Shonil Bhagwat, Ramón Cuevas Guzmán and two anonymous reviewers for their valuable comments and suggestions on the manuscript. Ramón Cuevas Guzmán and Luis Guzmán Hernández identified botanical material. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO) and the Universidad de Guadalajara provided funds for the fieldwork. The senior author was supported by Conacyt (Mexican National Council for Science and Technology) and Promep (SEP) through a Doctorate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olvera-Vargas, M., Figueroa-Rangel, B.L. & Vázquez-López, J.M. Is there environmental differentiation in the Quercus-dominated forests of west-central Mexico?. Plant Ecol 211, 321–335 (2010). https://doi.org/10.1007/s11258-010-9792-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-010-9792-z