Abstract
Purpose
Incremental peritoneal dialysis (IPD) could decrease unfavorable glucose exposure results and preserve (RKF). However, there is no standardization of dialysis prescriptions for patients undergoing IPD. We designed a prospective observational multi-center study with a standardized IPD prescription to evaluate the effect of IPD on RKF, metabolic alterations, blood pressure control, and adverse outcomes.
Methods
All patients used low GDP product (GDP) neutral pH solutions in both the incremental continuous ambulatory peritoneal dialysis (ICAPD) group and the retrospective standard PD (sPD) group. IPD patients started treatment with three daily exchanges five days a week. Control-group patients performed four changes per day, seven days a week.
Results
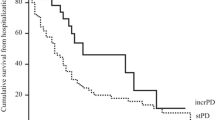
A total of 94 patients (47 IPD and 47 sPD) were included in this study. The small-solute clearance and mean blood pressures were similar between both groups during follow-up. The weekly mean glucose exposure was significantly higher in sPD group than IPD during the follow-up (p < 0.001). The patients with sPD required more phosphate-binding medications compared to the IPD group (p = 0.05). The rates of peritonitis, tunnel infection, and hospitalization frequencies were similar between groups. Patients in the sPD group experienced more episodes of hypervolemia compared to the IPD group (p = 0.007). The slope in RKF in the 6th month was significantly higher in the sPD group compared to the IPD group (65% vs. 95%, p = 0.001).
Conclusion
IPD could be a rational dialysis method and provide non-inferior dialysis adequacy compared to full-dose PD. This regimen may contribute to preserving RKF for a longer period.



Similar content being viewed by others
Data availability
The data could be participated in case of request.
References
Churchill DN, Thorpe KE, Vonesh EF, Keshaviah PR (1997) Lower probability of patient survival with continuous peritoneal dialysis in the United States compared with Canada. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 8:965–971
Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S, Group MNCS (2002) Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 13:1307–1320
Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT (2003) The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 41:1293–1302
Fernandes A, Matias P, Branco P (2023) Incremental peritoneal dialysis—definition, prescription, and clinical outcomes. Kidney360 4:272–277
Golper T, Churchill D, Burkart J, Firanek C, Geary D, Gotch F, Moore L, Nolph K, Powe N, Singh H (1997) NKF DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 30:S67-136
Dhoot A, Brown EA, Robinson B, Perl J (2023) Incremental peritoneal dialysis: incremental gains. SAGE Publications, London, pp 355–358
Brown EA, Blake PG, Boudville N, Davies S, de Arteaga J, Dong J, Finkelstein F, Foo M, Hurst H, Johnson DW (2020) International society for peritoneal dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int 40:244–253
Cheetham MS, Cho Y, Krishnasamy R, Jain AK, Boudville N, Johnson DW, Huang LL (2022) Incremental versus standard (full-dose) peritoneal dialysis. Kidney Int Rep 7:165–176
Auguste BL, Bargman JM (2018) Incremental peritoneal dialysis: new ideas about an old approach. Semin Dial 31:445–448
Nolph KO, Khanna R, Prowant BF, Ryan LP, Moore HL, Nielsen MP (1987) Peritoneal equilibration test. Perit Dial Int 7:138–148
Li PKT, Chow KM, Cho Y et al (2022) ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int 42(2):110–153
Hayat A, Cho Y, Hawley CM, Htay H, Krishnasamy R, Pascoe E, Teitelbaum I, Varnfield M, Johnson DW (2023) Association of incremental peritoneal dialysis with residual kidney function decline in patients on peritoneal dialysis: the balANZ trial. Perit Dial Int. https://doi.org/10.1177/08968608231175826
Cheetham MS, Cho Y, Krishnasamy R, Milanzi E, Chow J, Hawley C, Moodie J-A, Jose MD, MacGinley R, Nguyen T (2023) Multicentre registry analysis of incremental peritoneal dialysis incidence and associations with patient outcomes. Perit Dial Int 43:383–394
Sandrini M, Vizzardi V, Valerio F, Ravera S, Manili L, Zubani R, Lucca BJ, Cancarini G (2016) Incremental peritoneal dialysis: a 10 year single-centre experience. J Nephrol 29:871–879
Tomo T, Okabe E, Matsuyama K, Iwashita T, Yufu K, Nasu M (2005) The effect of peritoneal rest in combination therapy of peritoneal dialysis and hemodialysis: using the cultured human peritoneal mesothelial cell model. J Artif Organs 8:125–129
Ueda A, Nagai K, Yamagata K (2021) Preserved peritoneal function by short-term two-day peritoneal rest in hemodialysis combination therapy patients. J Artif Organs 24:296–300
Alrowiyti IM, Bargman J (2023) A review of residual kidney function in peritoneal dialysis patients. Indian J Nephrol 33:239–246
Menon MK, Naimark DM, Bargman JM, Vas SI, Oreopoulos DG (2001) Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transpl 16:2207–2213
Wang AY-M, Wang M, Woo J, Lam CW-K, Lui S-F, Li PK-T, Sanderson JE (2004) Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol 15:2186–2194
Rhee H, Yang JY, Jung WJ, Shin MJ, Yang BY, Song SH, Kwak IS, Seong EY (2014) Significance of residual renal function for phosphate control in chronic hemodialysis patients. Kidney Res Clin Pract 33:58–64
Funding
None.
Author information
Authors and Affiliations
Contributions
Hasan Haci Yeter: study design, writing, analysis. Murat Altunok: data collection. Erdem Cankaya: mentor of study design, critical review and paper correction. Saliha Yildirim: resource, data collection, study design. Serkan Akturk: data collection, study design. Serkan Bakirdogen: data collection. Hadim Akoglu: data collection, study design. Mesudiye Bulut: data collection. Tuncay Sahutoglu: data collection. Arda Erdut: data collection. Mehmet Ozkahya: data collection. Yener Koc: data collection. Onur Tunca: data collection. Ekrem Kara: data collection. Müge Erek: data collection. Mehmet Polat: data collection. Tulin Akagun: data collection. Galip Guz: mentor of study design, critical review and paper correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The design and procedures of the study were approved by the Ethics Committee of the University and Turkish Pharmaceuticals and Medical Devices Agency in agreement with the principles of the Declaration of Helsinki and ethical standards for human experimentation (Date:18/04/2022, No:331/Date: 08/09/2022 No: 88521274, respectively).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeter, H.H., Altunok, M., Cankaya, E. et al. Effects of incremental peritoneal dialysis with low glucose-degradation product neutral pH solution on clinical outcomes. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04077-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04077-7