Abstract
Purpose
Multiple circulating proteins have been reported to participate in human diseases. However, the association between cardiovascular proteins and membranous nephropathy (MN) remained profoundly elusive.
Methods
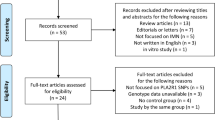
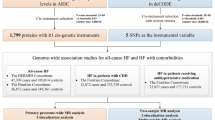
A bidirectional Mendelian randomization (MR) analysis was conducted to explore the causal correlation between ninety cardiovascular proteins and MN. Genome-wide association study (GWAS) data of cardiovascular proteins and MN were all from European research. Inverse variance weighted (IVW) was used as the main approach. Moreover, MR-Egger, weighted median, weighted mode, and simple mode were also performed. Cochrane’s Q test, MR-Egger, and MR-PRESSO were conducted for sensitivity analysis.
Results
According to IVW method, fatty acid-binding protein and thrombomodulin (TM) were identified as risk factors for MN, while a protective role was detected in tissue-type plasminogen activator. Additionally, MN was associated with an elevated level of macrophage colony-stimulating factor 1, stem cell factor, TM, and tissue factor. Reversely, MN was also correlated with a downregulated level of beta-nerve growth factor, Cathepsin D, hepatocyte growth factor, interleukin-6 receptor subunit alpha, macrophage colony-stimulating factor 1, and myeloperoxidase. In the sensitivity analysis, no significant pleiotropy and heterogeneity was detected.
Conclusion
This was the first study to reveal the causal association between cardiovascular proteins and MN. These specific cardiovascular proteins could be novel biomarkers for MN, and is helpful for timely identify the risk of other diseases that might result from MN. However, further clinical studies are needed to confirm our results.







Similar content being viewed by others
Data availability statement
The datasets presented in this study could be found in online site: https://gwas.mrcieu.ac.uk/ and Supplementary Material.
References
Ronco, P., L. Beck, H. Debiec, F. C. Fervenza, F. F. Hou, V. Jha, S. Sethi, A. Tong, M. Vivarelli and J. Wetzels. "Membranous nephropathy." Nat Rev Dis Primers. 2021. https://doi.org/10.1038/s41572-021-00303-z.
Gu, Y., H. Xu and D. Tang. "Mechanisms of primary membranous nephropathy." Biomolecules. 2021. https://doi.org/10.3390/biom11040513.
Xiang, M., Y. Wang, Z. Gao, J. Wang, Q. Chen, Z. Sun, J. Liang and J. Xu. "Exploring causal correlations between inflammatory cytokines and systemic lupus erythematosus: A mendelian randomization." Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.985729.
Groza, Y., J. Jemelkova, L. R. Kafkova, P. Maly and M. Raska. "Il-6 and its role in iga nephropathy development." Cytokine Growth Factor Rev. 2022: 1–14. https://doi.org/10.1016/j.cytogfr.2022.04.001.
Kondo, N., T. Kuroda and D. Kobayashi. "Cytokine networks in the pathogenesis of rheumatoid arthritis." Int J Mol Sci. 2021. https://doi.org/10.3390/ijms222010922.
Mezzano, S. A., M. A. Droguett, M. E. Burgos, L. G. Ardiles, C. A. Aros, I. Caorsi and J. Egido. "Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy." Kidney Int. 2000: 147–58. https://doi.org/10.1046/j.1523-1755.2000.00830.x.
Wootton, R. E., R. C. Richmond, B. G. Stuijfzand, R. B. Lawn, H. M. Sallis, G. M. J. Taylor, G. Hemani, H. J. Jones, S. Zammit, G. Davey Smith, et al. "Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: A mendelian randomisation study." Psychol Med. 2020: 2435–43. https://doi.org/10.1017/s0033291719002678.
Sekula, P., M. F. Del Greco, C. Pattaro and A. Köttgen. "Mendelian randomization as an approach to assess causality using observational data." J Am Soc Nephrol. 2016: 3253–65. https://doi.org/10.1681/asn.2016010098.
Hartwig, F. P., N. M. Davies, G. Hemani and G. Davey Smith. "Two-sample mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique." Int J Epidemiol. 2016: 1717–26. https://doi.org/10.1093/ije/dyx028.
Davey Smith, G. and G. Hemani. "Mendelian randomization: Genetic anchors for causal inference in epidemiological studies." Hum Mol Genet. 2014: R89–98. https://doi.org/10.1093/hmg/ddu328.
Boef, A. G., O. M. Dekkers and S. le Cessie. "Mendelian randomization studies: A review of the approaches used and the quality of reporting." Int J Epidemiol. 2015: 496–511. https://doi.org/10.1093/ije/dyv071.
Pritchard, J. K. and M. Przeworski. "Linkage disequilibrium in humans: Models and data." Am J Hum Genet. 2001: 1–14. https://doi.org/10.1086/321275.
Burgess, S. and S. G. Thompson. "Avoiding bias from weak instruments in mendelian randomization studies." Int J Epidemiol. 2011: 755–64. https://doi.org/10.1093/ije/dyr036.
Pierce, B. L., H. Ahsan and T. J. Vanderweele. "Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants." Int J Epidemiol. 2011: 740–52. https://doi.org/10.1093/ije/dyq151.
Folkersen, L., S. Gustafsson, Q. Wang, D. H. Hansen, K. Hedman Å, A. Schork, K. Page, D. V. Zhernakova, Y. Wu, J. Peters, et al. "Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals." Nat Metab. 2020: 1135–48. https://doi.org/10.1038/s42255-020-00287-2.
Xie, J., L. Liu, N. Mladkova, Y. Li, H. Ren, W. Wang, Z. Cui, L. Lin, X. Hu, X. Yu, et al. "The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis." Nat Commun. 2020. https://doi.org/10.1038/s41467-020-15383-w.
Cai, J., X. Li, S. Wu, Y. Tian, Y. Zhang, Z. Wei, Z. Jin, X. Li, X. Chen and W. X. Chen. "Assessing the causal association between human blood metabolites and the risk of epilepsy." J Transl Med. 2022: 437. https://doi.org/10.1186/s12967-022-03648-5.
Ren, F., Q. Jin, T. Liu, X. Ren and Y. Zhan. "Causal effects between gut microbiota and iga nephropathy: A bidirectional mendelian randomization study." Front Cell Infect Microbiol. 2023. https://doi.org/10.3389/fcimb.2023.1171517.
Verbanck, M., C. Y. Chen, B. Neale and R. Do. "Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases." Nat Genet. 2018: 693–98. https://doi.org/10.1038/s41588-018-0099-7.
Bowden, J., G. Davey Smith and S. Burgess. "Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression." Int J Epidemiol. 2015: 512–25. https://doi.org/10.1093/ije/dyv080.
Su, M., Y. Tang, W. Kong, S. Zhang and T. Zhu. "Genetically supported causality between gut microbiota, gut metabolites and low back pain: A two-sample mendelian randomization study." Front Microbiol. 2023. https://doi.org/10.3389/fmicb.2023.1157451.
Shrestha, S., H. Sunaga, H. Hanaoka, A. Yamaguchi, S. Kuwahara, Y. Umbarawan, K. Nakajima, T. Machida, M. Murakami, A. Saito, et al. "Circulating fabp4 is eliminated by the kidney via glomerular filtration followed by megalin-mediated reabsorption." Sci Rep. 2018. https://doi.org/10.1038/s41598-018-34902-w.
Shi, M., L. Ma and P. Fu. "Role of fatty acid binding protein 4 (fabp4) in kidney disease." Curr Med Chem. 2020: 3657–64. https://doi.org/10.2174/0929867325666181008154622.
Hofstra, J. M., J. K. Deegens, E. J. Steenbergen and J. F. Wetzels. "Urinary excretion of fatty acid-binding proteins in idiopathic membranous nephropathy." Nephrol Dial Transplant. 2008: 3160–5. https://doi.org/10.1093/ndt/gfn190.
van de Logt, A. E., M. Fresquet, J. F. Wetzels and P. Brenchley. "The anti-pla2r antibody in membranous nephropathy: What we know and what remains a decade after its discovery." Kidney Int. 2019: 1292–302. https://doi.org/10.1016/j.kint.2019.07.014.
Allyson J, Bi X, Baudry M, Massicotte G (2012) Maintenance of synaptic stability requires calcium-independent phospholipase a2 activity. Neural Plast. https://doi.org/10.1155/2012/569149
Giardino L, Armelloni S, Corbelli A, Mattinzoli D, Zennaro C, Guerrot D, Tourrel F, Ikehata M, Li M, Berra S et al (2009) Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J Am Soc Nephrol 20(9):1929–1940. https://doi.org/10.1681/asn.2008121286
Kerlin BA, Ayoob R, Smoyer WE (2012) Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 7:513–520. https://doi.org/10.2215/cjn.10131011
Li J, Wang X, Jiang S, Li W (2023) Serum pla2r antibody as a predictive biomarker for venous thromboembolism risk in primary membranous nephropathy. Med Clin (Barc). https://doi.org/10.1016/j.medcli.2023.06.007
Tomura S, Deguchi F, Marumo F, Aoki N (1994) Enhanced presence of thrombomodulin in the glomeruli of lupus glomerulonephritis. Clin Nephrol 41(4):205–210
Gue YX, Gorog DA (2017) Importance of endogenous fibrinolysis in platelet thrombus formation. Int J Mol Sci. https://doi.org/10.3390/ijms18091850
Takeshita A, Yasuma T, Nishihama K, D’Alessandro-Gabazza CN, Toda M, Totoki T, Okano Y, Uchida A, Inoue R, Qin L et al (2020) Thrombomodulin ameliorates transforming growth factor-β1-mediated chronic kidney disease via the g-protein coupled receptor 15/akt signal pathway. Kidney Int. https://doi.org/10.1016/j.kint.2020.05.041
Wu CC, Chen JS, Huang CF, Chen CC, Lu KC, Chu P, Sytwu HK, Lin YF (2011) Approaching biomarkers of membranous nephropathy from a murine model to human disease. J Biomed Biotechnol. https://doi.org/10.1155/2011/581928
Singh AK (1992) Proteolytic machinery of glomerular epithelial cells against igg. Biochem Biophys Res Commun 186(2):639–644. https://doi.org/10.1016/0006-291x(92)90794-l
Li L, He D, Yang J, Wang X (2011) Cordycepin inhibits renal interstitial myofibroblast activation probably by inducing hepatocyte growth factor expression. J Pharmacol Sci 117(4):286–294. https://doi.org/10.1254/jphs.11127fp
Funakoshi H, Nakamura T (2003) Hepatocyte growth factor: from diagnosis to clinical applications. Clin Chim Acta. https://doi.org/10.1016/s0009-8981(02)00302-9
Romero-Vásquez F, Chávez M, Pérez M, Arcaya JL, García AJ, Rincón J, Rodríguez-Iturbe B (2012) Overexpression of hgf transgene attenuates renal inflammatory mediators, na(+)-atpase activity and hypertension in spontaneously hypertensive rats. Biochim Biophys Acta. https://doi.org/10.1016/j.bbadis.2012.06.006
Esposito C, Parrilla B, De Mauri A, Cornacchia F, Fasoli G, Foschi A, Mazzullo T, Plati A, Scudellaro R, Dal Canton A (2005) Hepatocyte growth factor (hgf) modulates matrix turnover in human glomeruli. Kidney Int. https://doi.org/10.1111/j.1523-1755.2005.00319.x
Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, Sakkal S, Samuel CS, Ramsay RG, Deane JA et al (2011) Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol. https://doi.org/10.1016/j.ajpath.2011.05.037
Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC, Abboud Werner SL, Harris RC, Zhang MZ (2015) Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int. https://doi.org/10.1038/ki.2015.295
Stokman G, Stroo I, Claessen N, Teske GJ, Weening JJ, Leemans JC, Florquin S (2010) Stem cell factor expression after renal ischemia promotes tubular epithelial survival. PLoS ONE. https://doi.org/10.1371/journal.pone.0014386
Zhang W, Jia L, Liu DLX, Chen L, Wang Q, Song K, Nie S, Ma J, Chen X, Xiu M et al (2019) Serum stem cell factor level predicts decline in kidney function in healthy aging adults. J Nutr Health Aging. https://doi.org/10.1007/s12603-019-1253-3
Birner P, Heider S, Petzelbauer P, Wolf P, Kornauth C, Kuroll M, Merkel O, Steiner G, Kishimoto T, Rose-John S et al (2016) Interleukin-6 receptor alpha blockade improves skin lesions in a murine model of systemic lupus erythematosus. Exp Dermatol. https://doi.org/10.1111/exd.12934
Ndrepepa G (2019) Myeloperoxidase—a bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta. https://doi.org/10.1016/j.cca.2019.02.022
Tang J, Liao Z, Luo L, Deng S, Jiang Y, Wang F, Hu X, Yin H, Gong G, Feng J et al (2022) Cx3cl1-induced cd16+ monocytes extravasation in myeloperoxidase-anca-associated vasculitis correlates with renal damage. Front Immunol. https://doi.org/10.3389/fimmu.2022.929244
Hu ZJ, Niu K, Liu B, Shi YN (2014) A case of membranous nephropathy and myeloperoxidase anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Exp Ther Med. https://doi.org/10.3892/etm.2014.1852
Mapp PI, Walsh DA (2012) Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. https://doi.org/10.1038/nrrheum.2012.80
Acknowledgements
The authors would like to thank the participants and investigators of the GWAS used in this study.
Funding
This work was supported by the Key Clinical Research Project of the Second Affiliated Hospital of Nanchang University (No. 2022efyB01), the “Thousand Talents Plan” Project of Introducing and Training High‐level Talents of Innovation and Entrepreneurship in Jiangxi Province (No. JXSQ2023201030), and the Jiangxi Graduate Innovation Special Fund (No. YC2023-B090).
Author information
Authors and Affiliations
Contributions
Q. M. conducted conception, design, data collection, funding, and manuscript writing. G. X. was responsible for the funds and paper revision. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial interests.
Ethics statement
The study was conducted based on the data that have publicly available, so no additional ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Q., Xu, G. Causal association between cardiovascular proteins and membranous nephropathy: a bidirectional Mendelian randomization. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04004-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04004-w