Abstract
Purpose
The Geriatric Nutrition Risk Index (GNRI) is a simple and validated tool used to assess the nutritional status of elderly patients and predict the risk of short-term postoperative complications, as well as the long-term prognosis, after cancer surgery. In this study, we aimed to evaluate the predictive value of GNRI for the long-term postoperative prognosis in elderly patients with primary non-muscle-invasive bladder cancer (NMIBC) who underwent transurethral resection of bladder tumor (TURBT).
Methods
We retrospectively analyzed data from 292 elderly patients with primary NMIBC. Using X-tile software, we divided the cohort into two groups based on GNRI and determined the cut-off value for postoperative recurrence-free survival (RFS). Propensity score matching (PSM) with a ratio of 1:3, Kaplan–Meier analysis, log-rank test, and COX proportional hazards regression were used to assess the correlation between GNRI and prognosis and identify factors predicting recurrence and progression.
Results
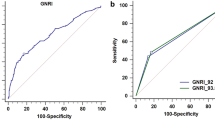
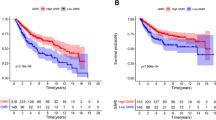
In the entire cohort, the 3 year recurrence group had significantly lower GNRI compared to the 3 year non-recurrence group (P = 0.0109). The determined GNRI cut-off value was 93.82. After PSM, the low GNRI group had significantly lower RFS (P < 0.0001) and progression-free survival (PFS) (P = 0.0040) than the high GNRI group. Multivariate COX regression showed that GNRI independently predicted RFS (HR 2.108; 95% CI 1.266–3.512; P = 0.004) and PFS (HR 2.155; 95% CI 1.135–4.091; P = 0.019) in elderly patients with primary NMIBC.
Conclusion
Preoperative GNRI is a prognostic marker for disease recurrence and progression in elderly patients with primary NMIBC undergoing TURBT.


Similar content being viewed by others
Data availability
The data supporting the results of this study were available at the corresponding author if reasonably requested.
References
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Babjuk M et al (2022) European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol 81(1):75–94. https://doi.org/10.1016/j.eururo.2021.08.010
Sylvester RJ et al (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49(3):466–475. https://doi.org/10.1016/j.eururo.2005.12.031
Constans T et al (1992) Protein-energy malnutrition in elderly medical patients. J Am Geriatr Soc 40(3):263–268. https://doi.org/10.1111/j.1532-5415.1992.tb02080.x
Alcorta MD et al (2018) The importance of serum albumin determination method to classify patients based on nutritional status. Clin Nutr ESPEN. 25:110–113. https://doi.org/10.1016/j.clnesp.2018.03.124
Ballmer PE (2001) Causes and mechanisms of hypoalbuminaemia. Clin Nutr 20(3):271–273. https://doi.org/10.1054/clnu.2001.0439
Sheinenzon A et al (2021) Serum albumin levels and inflammation. Int J Biol Macromol 184:857–862. https://doi.org/10.1016/j.ijbiomac.2021.06.140
Bouillanne O et al (2005) Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82(4):777–783. https://doi.org/10.1093/ajcn/82.4.777
Riveros C et al (2022) The geriatric nutritional risk index predicts postoperative outcomes in bladder cancer: a propensity score-matched analysis. J Urol 207(4):797–804. https://doi.org/10.1097/ju.0000000000002342
Riveros C et al (2023) The geriatric nutritional risk index predicts complications after nephrectomy for renal cancer. Int Braz J Urol 49(1):97–109. https://doi.org/10.1590/s1677-5538.Ibju.2022.0380
Pan Y, Zhong X, Liu J (2022) The prognostic significance of Geriatric nutritional risk index in elderly patients with bladder cancer after radical cystectomy. Asian J Surg. https://doi.org/10.1016/j.asjsur.2022.11.082
Kanno H et al (2021) Geriatric nutritional risk index predicts prognosis in hepatocellular carcinoma after hepatectomy: a propensity score matching analysis. Sci Rep 11(1):9038. https://doi.org/10.1038/s41598-021-88254-z
Lu S et al (2023) The preoperative geriatric nutritional risk index predicts long-term prognosis in elderly locally advanced rectal cancer patients: a two-center retrospective cohort study. Aging Clin Exp Res 35(2):311–321. https://doi.org/10.1007/s40520-022-02297-4
Takahashi M et al (2021) Comparison of three nutritional scoring systems for outcomes after complete resection of non-small cell lung cancer. J Thorac Cardiovasc Surg 162(4):1257-1268.e3. https://doi.org/10.1016/j.jtcvs.2020.06.030
Wyszynski A et al (2014) Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer 120(3):408–414. https://doi.org/10.1002/cncr.28394
Babjuk M et al (2017) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 71(3):447–461. https://doi.org/10.1016/j.eururo.2016.05.041
Niwa N et al (2015) Comparison of outcomes between ultrasonography and cystoscopy in the surveillance of patients with initially diagnosed TaG1-2 bladder cancers: a matched-pair analysis. Urol Oncol 33(9):386 e15-421. https://doi.org/10.1016/j.urolonc.2015.04.018
Nakagawa N, Maruyama K, Hasebe N (2021) Utility of geriatric nutritional risk index in patients with chronic kidney disease: a mini-review. Nutrients. https://doi.org/10.3390/nu13113688
Li H et al (2021) Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res 33(6):1477–1486. https://doi.org/10.1007/s40520-020-01656-3
Maenosono R et al (2022) Unplanned hemodialysis initiation and low geriatric nutritional risk index scores are associated with end-stage renal disease outcomes. Sci Rep 12(1):11101. https://doi.org/10.1038/s41598-022-14123-y
Yenibertiz D, Cirik MO (2021) The comparison of GNRI and other nutritional indexes on short-term survival in geriatric patients treated for respiratory failure. Aging Clin Exp Res 33(3):611–617. https://doi.org/10.1007/s40520-020-01740-8
Wang PY et al (2021) Application of four nutritional risk indexes in perioperative management for esophageal cancer patients. J Cancer Res Clin Oncol 147(10):3099–3111. https://doi.org/10.1007/s00432-021-03585-8
Norman K et al (2008) Prognostic impact of disease-related malnutrition. Clin Nutr 27(1):5–15. https://doi.org/10.1016/j.clnu.2007.10.007
Zheng HL et al (2017) Effects of preoperative malnutrition on short- and long-term outcomes of patients with gastric cancer: can we do better? Ann Surg Oncol 24(11):3376–3385. https://doi.org/10.1245/s10434-017-5998-9
Nicolini A et al (2013) Malnutrition, anorexia and cachexia in cancer patients: a mini-review on pathogenesis and treatment. Biomed Pharmacother 67(8):807–817. https://doi.org/10.1016/j.biopha.2013.08.005
Zhang X, Edwards BJ (2019) Malnutrition in older adults with cancer. Curr Oncol Rep 21(9):80. https://doi.org/10.1007/s11912-019-0829-8
Baracos VE et al (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4:17105. https://doi.org/10.1038/nrdp.2017.105
Pamoukdjian F et al (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 37(4):1101–1113. https://doi.org/10.1016/j.clnu.2017.07.010
Shoji F et al (2017) Relationship between preoperative sarcopenia status and immuno-nutritional parameters in patients with early-stage non-small cell lung cancer. Anticancer Res 37(12):6997–7003. https://doi.org/10.21873/anticanres.12168
Cereda E, Vanotti A (2007) The new Geriatric Nutritional Risk Index is a good predictor of muscle dysfunction in institutionalized older patients. Clin Nutr 26(1):78–83. https://doi.org/10.1016/j.clnu.2006.09.007
Arthuso FZ et al (2022) Associations between body mass index and bladder cancer survival: is the obesity paradox short-lived? Can Urol Assoc J 16(5):E261-e267. https://doi.org/10.5489/cuaj.7546
Evers J et al (2020) No clear associations of adult BMI and diabetes mellitus with non-muscle invasive bladder cancer recurrence and progression. PLoS One 15(3):e0229384. https://doi.org/10.1371/journal.pone.0229384
Lai CC et al (2011) Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis 26(4):473–481. https://doi.org/10.1007/s00384-010-1113-4
Evans DC et al (2021) The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract 36(1):22–28. https://doi.org/10.1002/ncp.10588
Yeh SS, Schuster MW (1999) Geriatric cachexia: the role of cytokines. Am J Clin Nutr 70(2):183–197. https://doi.org/10.1093/ajcn.70.2.183
Huang Y et al (1995) Interleukin-6 down-regulates expressions of the aldolase B and albumin genes through a pathway involving the activation of tyrosine kinase. Arch Biochem Biophys 320(2):203–209. https://doi.org/10.1016/0003-9861(95)90001-2
Li J et al (2018) The association of pretreatment serum albumin with outcomes in bladder cancer: a meta-analysis. Onco Targets Ther 11:3449–3459. https://doi.org/10.2147/OTT.S162066
Onate-Ocana LF et al (2007) Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol 14(2):381–389. https://doi.org/10.1245/s10434-006-9093-x
Asher V, Lee J, Bali A (2011) Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol 29(3):2005–2009. https://doi.org/10.1007/s12032-011-0019-5
Funding
No funds, grants, or other support were received.
Author information
Authors and Affiliations
Contributions
The design of this study, data collection, and writing of the manuscript were done by JXW, XFC, and HY. LHX, SX, and CWJ: contributed to the follow-up and data analysis. WH, CZ, and GXW: supervised the whole project, discussed the analysis results, and reviewed the manuscript, All authors participated in the final review of the manuscript and agreed to submit the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Review Board of the First Affiliated Hospital of Nanchang University.
Consent to participate
All clinical data come from the inpatient system and the outpatient system, so the consent of the patient is no longer required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Cheng, X., Yang, H. et al. Geriatric nutritional risk index as a prognostic factor in elderly patients with non-muscle-invasive bladder cancer: a propensity score-matched study. Int Urol Nephrol 56, 1627–1637 (2024). https://doi.org/10.1007/s11255-023-03905-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03905-6