Abstract
Purpose
To investigate the effect of low-intensity extracorporeal shock wave therapy (LiESWT) on lipopolysaccharide (LPS)-induced cystitis in an animal model of interstitial cystitis/bladder pain syndrome (IC/BPS).
Methods
Sprague–Dawley rats were divided into three groups: control, cystitis (LPS group, intravesical injection of LPS (1 mg) twice), and cystitis with LiESWT (LiESWT group). On the third and fourth days, LiESWT was administered (0.12 mJ/mm2, 300 shots each time) on the lower abdomen toward the bladder. On the seventh day, the rats underwent pain assessment and a metabolic cage study. Subsequently, a continuous cystometrogram (CMG) was performed under urethane anaesthesia. Immunohistochemical studies were also performed, including S-100 staining, an immunohistochemical marker of Schwann cells in the bladder.
Results
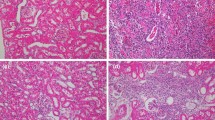
In the LPS group, the pain threshold in the lower abdomen was significantly lower than that in the control group. In the metabolic cage study, the mean voided volume in the LPS group significantly increased. The CMG also revealed a significant decrease in bladder contraction amplitude, compatible with detrusor underactivity in the LPS group. Immunohistochemical studies showed inflammatory changes in the submucosa, increased fibrosis, and decreased S-100 stain-positive areas in the muscle layer of the LPS group. In the LiESWT group, tactile allodynia and bladder function were ameliorated, and S-100 stain-positive areas were increased.
Conclusion
By restoring nerve damage, LiESWT improved lower abdominal pain sensitivity and bladder function in an LPS-induced cystitis rat model. This study suggests that LiESWT may be a new therapeutic modality for IC/BPS.





Similar content being viewed by others
Availability of data and material
The data sets of the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Akiyama Y, Luo Y, Hanno PM, Maeda D, Homma Y (2020) Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol 27:491–503. https://doi.org/10.1111/iju.14229
Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK et al (2010) Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: a case control study. J Urol 183:167–172. https://doi.org/10.1016/j.juro.2009.08.133
Wu EQ, Birnbaum H, Mareva M, Parece A, Huang Z, Mallett D et al (2006) Interstitial cystitis: cost, treatment and co-morbidities in an employed population. Pharmacoeconomics 24:55–65. https://doi.org/10.2165/00019053-200624010-00005
Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P et al (2011) Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 186:540–544. https://doi.org/10.1016/j.juro.2011.03.132
Whitmore KE, Fall M, Sengiku A, Tomoe H, Logadottir Y, Kim YH (2019) Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int J Urol 26(Suppl 1):26–34. https://doi.org/10.1111/iju.13971
Song PH, Chun SY, Chung J-W, Kim YY, Lee HJ, Lee JN et al (2017) Comparison of 5 different rat models to establish a standard animal model for research into interstitial cystitis. Int Neurourol J 21:163–170. https://doi.org/10.5213/inj.1734898.449
Yoshizumi M, Watanabe C, Mizoguchi H (2021) Gabapentin reduces painful bladder hypersensitivity in rats with lipopolysaccharide-induced chronic cystitis. Pharmacol Res Perspect 9:e00697. https://doi.org/10.1002/prp2.697
Kim A, Han J-Y, Ryu C-M, Yu HY, Lee S, Kim Y et al (2017) Histopathological characteristics of interstitial cystitis/bladder pain syndrome without Hunner lesion. Histopathology 71:415–424. https://doi.org/10.1111/his.13235
Ryu C-M, Shin JH, Yu HY, Ju H, Kim S, Lim J et al (2019) N-acetylcysteine prevents bladder tissue fibrosis in a lipopolysaccharide-induced cystitis rat model. Sci Rep 9:8134. https://doi.org/10.1038/s41598-019-44631-3
Gerdesmeyer L, Wagenpfeil S, Haake M, Maier M, Loew M, Wörtler K et al (2003) Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA 290:2573–2580. https://doi.org/10.1001/jama.290.19.2573
Kikuchi Y, Ito K, Ito Y, Shiroto T, Tsuburaya R, Aizawa K et al (2010) Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ J 74:589–591. https://doi.org/10.1253/circj.cj-09-1028
Kitrey ND, Gruenwald I, Appel B, Shechter A, Massarwa O, Vardi Y (2016) Penile Low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: a double-blind. Sham Controlled Study J Urol 195:1550–1555. https://doi.org/10.1016/j.juro.2015.12.049
Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H (2009) Extracorporeal shock wave therapy in inflammatory diseases: molecular mechanism that triggers anti-inflammatory action. Curr Med Chem 16:2366–2372
Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K et al (2004) Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation 110:3055–3061. https://doi.org/10.1161/01.CIR.0000148849.51177.97
Wang H-J, Tyagi P, Chen Y-M, Chancellor MB, Chuang Y-C (2019) Low energy shock wave therapy inhibits inflammatory molecules and suppresses prostatic pain and hypersensitivity in a capsaicin induced prostatitis model in rats. Int J Mol Sci. https://doi.org/10.3390/ijms20194777
Wang HS, Oh BS, Wang B, Ruan Y, Zhou J, Banie L et al (2018) Low-intensity extracorporeal shockwave therapy ameliorates diabetic underactive bladder in streptozotocin-induced diabetic rats. BJU Int 122:490–500. https://doi.org/10.1111/bju.14216
Kato K, Satoh T (1983) Changes in the concentration of enolase isozymes and S-100 protein in degenerating and regenerating rat sciatic nerve. J Neurochem 40:1076–1081
Zhang X, Yan X, Wang C, Tang T, Chai Y (2014) The dose-effect relationship in extracorporeal shock wave therapy: the optimal parameter for extracorporeal shock wave therapy. J Surg Res 186:484–492. https://doi.org/10.1016/j.jss.2013.08.013
Lin K-L, Lu J-H, Chueh K-S, Juan T-J, Wu B-N, Chuang S-M et al (2021) Low-Intensity extracorporeal shock wave therapy promotes bladder regeneration and improves overactive bladder induced by ovarian hormone deficiency from rat animal model to human clinical trial. Int J Mol Sci. https://doi.org/10.3390/ijms22179296
Bradman MJG, Ferrini F, Salio C, Merighi A (2015) Practical mechanical threshold estimation in rodents using von Frey hairs/Semmes-Weinstein monofilaments: towards a rational method. J Neurosci Methods 255:92–103. https://doi.org/10.1016/j.jneumeth.2015.08.010
Park BS, Lee J-O (2013) Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 45:e66. https://doi.org/10.1038/emm.2013.97
Kimura R, Miyazato M, Ashikari A, Oshiro T, Saito S (2018) Age-associated urethral dysfunction in urethane-anesthetized rats. Neurourol Urodyn 37:1313–1319. https://doi.org/10.1002/nau.23481
Otsubo A, Miyazato M, Oshiro T, Kimura R, Matsuo T, Miyata Y et al (2021) Age-associated bladder and urethral coordination impairment and changes in urethral oxidative stress in rats. Life Sci 279:119690. https://doi.org/10.1016/j.lfs.2021.119690
Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann B, Walther V (1982) First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol 127:417–420. https://doi.org/10.1016/s0022-5347(17)53841-0
Miyazato M, Yoshimura N, Chancellor MB (2013) The other bladder syndrome: underactive bladder. Rev Urol 15:11–22
Osman NI, Esperto F, Chapple CR (2018) Detrusor underactivity and the underactive bladder: a systematic review of preclinical and clinical studies. Eur Urol 74:633–643. https://doi.org/10.1016/j.eururo.2018.07.037
Chuang Y-C, Tyagi P, Wang H-J, Huang C-C, Lin C-C, Chancellor MB (2018) Urodynamic and molecular characteristics of detrusor underactivity in a rat cryoinjury model and effects of low energy shock wave therapy. Neurourol Urodyn 37:708–715. https://doi.org/10.1002/nau.23381
Lee Y-C, Hsieh T-J, Tang F-H, Jhan J-H, Lin K-L, Juan Y-S et al (2021) Therapeutic effect of low intensity extracorporeal shock wave therapy (Li-ESWT) on diabetic bladder dysfunction in a rat model. Int J Med Sci 18:1423–1431. https://doi.org/10.7150/ijms.55274
Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, Lue TF et al (2013) Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 10:738–746. https://doi.org/10.1111/jsm.12024
Wang B, Ning H, Reed-Maldonado AB, Zhou J, Ruan Y, Zhou T et al (2017) Low-intensity extracorporeal shock wave therapy enhances brain-derived neurotrophic factor expression through PERK/ATF4 signaling pathway. Int J Mol Sci. https://doi.org/10.3390/ijms18020433
Cui Z, Lv Q, Yan M, Cheng C, Guo Z, Yang J et al (2011) Elevated expression of CAPON and neuronal nitric oxide synthase in the sciatic nerve of rats following constriction injury. Vet J 187:374–380. https://doi.org/10.1016/j.tvjl.2010.01.014
Sharma HS, Nyberg F, Westman J, Alm P, Gordh T, Lindholm D (1998) Brain derived neurotrophic factor and insulin like growth factor-1 attenuate upregulation of nitric oxide synthase and cell injury following trauma to the spinal cord. An immunohistochemical study in the rat. Amino Acids 14:121–129. https://doi.org/10.1007/BF01345252
Yang C-H, Yip H-K, Chen H-F, Yin T-C, Chiang JY, Sung P-H et al (2019) Long-term therapeutic effects of extracorporeal shock wave-assisted melatonin therapy on mononeuropathic pain in rats. Neurochem Res 44:796–810. https://doi.org/10.1007/s11064-018-02713-0
Wang H-J, Tyagi P, Lin T-K, Huang C-C, Lee W-C, Chancellor MB et al (2022) Low energy shock wave therapy attenuates mitochondrial dysfunction and improves bladder function in HCl induced cystitis in rats. Biomed J 45:482–490. https://doi.org/10.1016/j.bj.2021.06.006
Li H, Zhang Z, Peng J, Xin Z, Li M, Yang B et al (2019) Treatment with low-energy shock wave alleviates pain in an animal model of uroplakin 3A-induced autoimmune interstitial cystitis/painful bladder syndrome. Investig Clin Urol 60:359–366. https://doi.org/10.4111/icu.2019.60.5.359
Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological Association (2015) Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 193:1545–1553. https://doi.org/10.1016/j.juro.2015.01.086
Chuang Y-C, Meng E, Chancellor M, Kuo H-C (2020) Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome-A prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol Urodyn 39:1505–1514. https://doi.org/10.1002/nau.24382
Acknowledgements
The authors express their gratitude to Hitoshi Uehara for assisting in preparing our experiments, Hiroyuki Arakawa for providing technical advice on pain assessment, and Ayako Nakaza for offering technical advice on immunohistochemistry. Additionally, we extend our appreciation to DOCTOR’s KITS Co., Ltd. for providing the ED-1000 and to Editage (www.editage.com) for their English language editing services.
Funding
This work was supported by AMED under Grant Number JP22gk0210025 and JSPS KAKENHI Grant Number 22K11420.
Author information
Authors and Affiliations
Contributions
The contributions of each author are as follows: (1) Substantial contributions to conception, experimental design, and investigation: NK, TCK, and MM. (2) Drafting and revising the article critically for important intellectual content: NK, TCK, NW, HC, NS, and MM. (3) Final approval of the version to be published: NK, TCK, NW, HC, NS, and MM. All contributors not meeting these criteria for authorship should instead be listed in the acknowledgements section.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study protocol was approved by the University of Ryukyu Institutional Animal Care and Use Committee (protocol no. A2020077, A2020019) in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kusakabe, N., Kamijo, T.C., Wada, N. et al. Effects of low-intensity extracorporeal shock wave therapy on lipopolysaccharide cystitis in a rat model of interstitial cystitis/bladder pain syndrome. Int Urol Nephrol 56, 77–86 (2024). https://doi.org/10.1007/s11255-023-03770-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03770-3