Abstract
Purpose
To investigate the effectiveness and safety of device-assisted intravesical chemotherapy compared to Bacillus Calmette–Guerin (BCG) in the treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC).
Methods
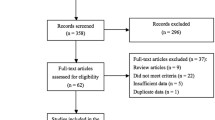
In February 2023, a systematic search was conducted on the PubMed, Cochrane, and Embase databases. Following the PRISMA guidelines, a systematic review and meta-analysis of the primary outcomes of interest were performed. The review was prospectively registered on PROSPERO under the registration number CRD42023398559.
Results
A total of 10 studies involving 1160 patients were included. The results of the meta-analysis showed that compared to BCG, device-assisted chemotherapy had a lower recurrence rate (OR: 0.63, 95% CI: 0.48–0.84, p = 0.001), longer recurrence-free survival (OR: 0.64, 95% CI: 0.47–0.88, p = 0.006), and lower incidence of fever (OR: 0.18, 95% CI: 0.08–0.44, p = 0.0002). However, no significant differences were observed between the two groups in terms of progression, overall survival, progression-free survival, disease-free survival, overall adverse events, serious adverse events, hematuria, allergy, and general discomfort. Subgroup analysis revealed that neither chemohyperthermia (CHT) nor electromotive drug administration (EMDA) showed statistically significant differences in oncological outcomes compared to BCG. Regarding adverse events, both CHT and EMDA groups showed lower rates of fever compared to the BCG group (OR: 0.26, 95% CI: 0.10–0.67, p = 0.005, and OR: 0.14, 95% CI: 0.05–0.37, p < 0.0001, respectively). No significant differences were observed in the remaining adverse events between either the CHT or EMDA group and the BCG group.
Conclusion
Device-assisted intravesical chemotherapy appears to be a safe and viable alternative to BCG for patients with intermediate and high-risk NMIBC, showing comparable oncological outcomes and adverse events.





Similar content being viewed by others
Data availability
All data generated and analyzed during this study are included in this published article.
References
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs—part B: prostate and bladder tumours. Eur Urol 70(1):106–119. https://doi.org/10.1016/j.eururo.2016.02.028
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Sylvester RJ, Brausi MA, Kirkels WJ, Hoeltl W, Calais Da Silva F, Powell PH, Prescott S, Kirkali Z, van de Beek C, Gorlia T, de Reijke TM, Group EG-UTC (2010) Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette–Guerin, and bacillus Calmette–Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 57(5): 766–773. https://doi.org/10.1016/j.eururo.2009.12.024
Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Roupret M, Shariat SF, Seisen T, Soukup V, Sylvester RJ (2022) European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol 81(1):75–94. https://doi.org/10.1016/j.eururo.2021.08.010
Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, Lotan Y, Meeks JJ, Michalski JM, Morgan TM, Quale DZ, Rosenberg JE, Zietman AL, Holzbeierlein JM (2017) Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol 198(3):552–559. https://doi.org/10.1016/j.juro.2017.04.086
Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, Necchi A, Valderrama BP, Ravaud A, Shariat SF, Szabados B, van der Heijden MS, Gillessen S, clinicalguidelines@esmo.org EGCEa (2022) Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 33(3):244–258. https://doi.org/10.1016/j.annonc.2021.11.012
Ojea A, Nogueira JL, Solsona E, Flores N, Gomez JM, Molina JR, Chantada V, Camacho JE, Pineiro LM, Rodriguez RH, Isorna S, Blas M, Martinez-Pineiro JA, Madero R, Group C (2007) A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette–Guerin (27 mg) versus very low-dose bacillus Calmette–Guerin (13.5 mg) versus mitomycin C. Eur Urol 52(5): 1398–1406. https://doi.org/10.1016/j.eururo.2007.04.062
Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FC, Oosterlinck W, Prescott S, Kirkali Z, Powell PH, de Reijke TM, Turkeri L, Collette S, Oddens J (2016) EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette–Guerin. Eur Urol 69(1):60–69. https://doi.org/10.1016/j.eururo.2015.06.045
Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA, Venema PL, Pollifrone DL, Harbrecht JD, Chin JL, Lerner SP, Miles BJ, International Mitomycin CC (2001) Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst 93(8): 597–604. https://doi.org/10.1093/jnci/93.8.597
You C, Li X, Du Y, Wang H, Zhang X, Wei T, Wang A (2021) Application of intra-arterial chemotherapy in high-risk non-muscle invasive bladder cancer: a systematic review and meta-analysis. PeerJ 9:e12248. https://doi.org/10.7717/peerj.12248
Zhao H, Chan VW, Castellani D, Chan EO, Ong WLK, Peng Q, Moschini M, Krajewski W, Pradere B, Ng CF, Enikeev D, Vasdev N, Ekin G, Sousa A, Leon J, Guerrero-Ramos F, Tan WS, Kelly J, Shariat SF, Witjes JA, Teoh JY (2021) Intravesical chemohyperthermia vs. bacillus Calmette–Guerin instillation for intermediate- and high-risk non-muscle invasive bladder cancer: a systematic review and meta-analysis. Front Surg 8:775527. https://doi.org/10.3389/fsurg.2021.775527
Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, Colombo R (2011) The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol 60(1):81–93. https://doi.org/10.1016/j.eururo.2011.04.023
Di Stasi SM, Riedl C (2009) Updates in intravesical electromotive drug administration of mitomycin-C for non-muscle invasive bladder cancer. World J Urol 27(3):325–330. https://doi.org/10.1007/s00345-009-0389-x
Jung JH, Gudeloglu A, Kiziloz H, Kuntz GM, Miller A, Konety BR, Dahm P (2017) Intravesical electromotive drug administration for non-muscle invasive bladder cancer. Cochrane Database Syst Rev 9 (9):CD011864. https://doi.org/10.1002/14651858.CD011864.pub2
Melgarejo-Segura MT, Morales-Martinez A, Yanez-Castillo Y, Arrabal-Polo MA, Gomez-Lechuga P, Pareja-Vilchez M, Jimenez-Moleon JJ, Martin MA (2023) A systematic review of the efficacy of intravesical electromotive drug administration therapy for non-muscle invasive bladder cancer. Urol Oncol 41(4):166–176. https://doi.org/10.1016/j.urolonc.2022.09.016
Carando R, Soldini E, Cotrufo S, Zazzara M, Ludovico GM (2020) Electro-mediated drug administration of mitomycin C in preventing non-muscle-invasive bladder cancer recurrence and progression after transurethral resection of the bladder tumour in intermediate- and high-risk patients. Arab J Urol 19(1):71–77. https://doi.org/10.1080/2090598X.2020.1816150
Tan WS, Prendergast A, Ackerman C, Yogeswaran Y, Cresswell J, Mariappan P, Phull J, Hunter-Campbell P, Lazarowicz H, Mishra V, Rane A, Davies M, Warburton H, Cooke P, Mostafid H, Wilby D, Mills R, Issa R, Kelly JD (2023) Adjuvant intravesical chemohyperthermia versus passive chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer (HIVEC-II): a phase 2, open-label, randomised controlled trial. Eur Urol 83(6):497–504. https://doi.org/10.1016/j.eururo.2022.08.003
Arends TJ, Nativ O, Maffezzini M, de Cobelli O, Canepa G, Verweij F, Moskovitz B, van der Heijden AG, Witjes JA (2016) Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus Calmette–Guérin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol 69(6):1046–1052. https://doi.org/10.1016/j.eururo.2016.01.006
Félix G-R, Daniel AG-P, Alejandro G-D, Federico de la R-K, Alfredo R-A, Brant AI, Felipe V-A (2022) Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial. World J Urol 40(4):999–1004. https://doi.org/10.1007/s00345-022-03928-1
Zazzara M, Nazaraj A, Scarcia M, Cardo G, Carando R, Ludovico GM (2023) Electromotive drug administration of mitomycin C (EMDA/MMC) versus intravesical immunotherapy with bacillus Calmette–Guérin (BCG) in intermediate and high risk non muscle invasive bladder cancer. Urol Int 107(1):64–71. https://doi.org/10.1159/000520630
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM, O’Mara AM, Li Y, Clauser SB, Bryant DM, Bearden JD 3rd, Gillis TA, Harness JK, Siegel RD, Paul DB, Cleeland CS, Schrag D, Sloan JA, Abernethy AP, Bruner DW, Minasian LM, Basch E, National Cancer Institute PROCSG (2015) Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol 1(8):1051–1059. https://doi.org/10.1001/jamaoncol.2015.2639
Clark HD, Wells GA, Huet C, McAlister FA, Salmi LR, Fergusson D, Laupacis A (1999) Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials 20(5):448–452. https://doi.org/10.1016/s0197-2456(99)00026-4
Wells GA, Shea B, O'Connell D et al (2000) The Newcastle Ottawa 1 Scale(NOS) for assessing the quality of nonrandomized studies in metaanalyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G (2011) The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Arrabal Polo MA, Melgarejo Segura MT, Yanez Castillo Y, Morales Martinez A, Pareja Vilchez M, Arrabal Martin M (2023) Adjuvant intravesical treatment in patients with intermediate and high-risk non-muscle-invasive bladder cancer with BCG versus MMC applied with COMBAT or EMDA. Results of a prospective study. J Cancer Res Clin Oncol 1–7. https://doi.org/10.1007/s00432-023-04688-0
Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Navarra P, Massoud R, Vespasiani G (2003) Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol 170(3):777–782. https://doi.org/10.1097/01.ju.0000080568.91703.18
Ekin RG, Akarken I, Zorlu F, Tarhan H, Kucuk U, Yildirim Z, Divrik RT (2015) Intravesical bacillus Calmette–Guerin versus chemohyperthermia for high-risk non-muscle-invasive bladder cancer. Can Urol Assoc J 9(5–6):E278-283. https://doi.org/10.5489/cuaj.2708
Kostyev F, Bondar O, Chystiakov R, Lysenko V, Stavnychyi O, Varbanets V (2021) The impact of different adjuvant intravesical therapy methods on tumor biology in patients with high-risk non-muscle-invasive bladder cancer. Central Eur J Urol 74(4):496–502. https://doi.org/10.5173/ceju.2021.0122
Tan WS, Panchal A, Buckley L, Devall AJ, Loubiere LS, Pope AM, Feneley MR, Cresswell J, Issa R, Mostafid H, Madaan S, Bhatt R, McGrath J, Sangar V, Griffiths TRL, Page T, Hodgson D, Datta SN, Billingham LJ, Kelly JD (2019) Radiofrequency-induced thermo-chemotherapy effect versus a second course of bacillus Calmette–Guerin or institutional standard in patients with recurrence of non-muscle-invasive bladder cancer following induction or maintenance bacillus Calmette–Guerin therapy (HYMN): a phase III, open-label, randomised controlled trial. Eur Urol 75(1):63–71. https://doi.org/10.1016/j.eururo.2018.09.005
Wang S, Yu Z, Du P, Cao Y, Yang X, Ma J, Tang X, Zhang Q, Yang Y (2023) Combination of hyperthermia and intravesical chemotherapy for the treatment of pT1 stage bladder cancer: a retrospectively clinical study. Asia Pac J Clin Oncol. https://doi.org/10.1111/ajco.13931
Yuvaraja BT, Santosh SW, Preetham D, Ashish A, Abhinav PP, Nevitha A, Abhijit R, Archan K, Naresh B (2021) Comparing adverse effects, short term outcomes, and cost implications of hyperthermic intravesical chemotherapy with mitomycin-C and intravesical bacillus Calmette–Guerin instillation (Moscow-I strain) in the management of intermediate and high-risk nonmuscle invasive bladder cancer. Urol Ann 13(4):424–430. https://doi.org/10.4103/ua.Ua_139_20
Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Pineiro L, Gonzalez M, Portillo J, Ojea A, Pertusa C, Rodriguez-Molina J, Camacho JE, Rabadan M, Astobieta A, Montesinos M, Isorna S, Muntanola P, Gimeno A, Blas M, Martinez-Pineiro JA (2009) Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette–Guerin: the CUETO scoring model. J Urol 182(5):2195–2203. https://doi.org/10.1016/j.juro.2009.07.016
Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49(3):466–465; discussion 475–467. https://doi.org/10.1016/j.eururo.2005.12.031
Carando R, Zazzara M, Cotrufo S, Ludovico GM (2019) Intravesical treatment with electro-mediated administration of mitomycin C as prophylaxis for intermediate and high-risk nonmuscle-invasive bladder cancer: a retrospective multicenter study. Urol Int 103(3):285–290. https://doi.org/10.1159/000502663
Wang C, Jin W, Ma X, Dong Z (2022) The different predictive value of mean platelet volume-to-lymphocyte ratio for postoperative recurrence between non-muscular invasive bladder cancer patients treated with intravesical chemotherapy and intravesical chemohyperthermia. Front Oncol 12:1101830. https://doi.org/10.3389/fonc.2022.1101830
Zargar H, Aning J, Ischia J, So A, Black P (2014) Optimizing intravesical mitomycin C therapy in non-muscle-invasive bladder cancer. Nat Rev Urol 11(4):220–230. https://doi.org/10.1038/nrurol.2014.52
Rampersaud EN, Vujaskovic Z, Inman BA (2010) Hyperthermia as a treatment for bladder cancer. Oncology (Williston Park) 24(12):1149–1155
Dewey WC, Westra A, Miller HH, Nagasawa H (1971) Heat-induced lethality and chromosomal damage in synchronized Chinese hamster cells treated with 5-bromodeoxyuridine. Int J Radiat Biol Relat Stud Phys Chem Med 20(6):505–520. https://doi.org/10.1080/09553007114551421
Colombo R, Da Pozzo LF, Lev A, Salonia A, Rigatti P, Leib Z, Servadio C, Caldarera E, Pavone-Macaluso M (1998) Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. J Urol 159(3):783–787
Carando R, Pradere B, Afferi L, Marra G, Aziz A, Roghmann F, Krajewski W, Di Bona C, Alvarez-Maestro M, Pagliarulo V, Xylinas E, Moschini M (2020) The role of device-assisted therapies in the management of non-muscle invasive bladder cancer: a systematic review. Prog Urol 30(6):322–331. https://doi.org/10.1016/j.purol.2020.03.005
Lu JL, Xia QD, Lu YH, Liu Z, Zhou P, Hu HL, Wang SG (2020) Efficacy of intravesical therapies on the prevention of recurrence and progression of non-muscle-invasive bladder cancer: a systematic review and network meta-analysis. Cancer Med 9(21):7800–7809. https://doi.org/10.1002/cam4.3513
Yamamoto S, Kageyama Y, Fujii Y, Aizawa T, Urakami S, Fukui I (2020) Randomized study of postoperative single intravesical instillation with pirarubicin and mitomycin C for low-risk bladder cancer. Anticancer Res 40(9):5295–5299. https://doi.org/10.21873/anticanres.14535
Johnson DC, Pruthi RS, Woods ME (2013) Perioperative chemotherapy: when to use it, what to use, and why. Urol Clin North Am 40(2):183–195. https://doi.org/10.1016/j.ucl.2013.01.001
Okamura T, Akita H, Ando R, Ikegami Y, Naiki T, Kawai N, Tozawa K, Kohri K (2012) Single monthly bacillus Calmette–Guerin intravesical instillation is effective maintenance therapy to prevent recurrence in Japanese patients with non-muscle-invasive bladder cancer. Int J Clin Oncol 17(5):477–481. https://doi.org/10.1007/s10147-011-0314-3
Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J, Roupret M, European Association of U (2013) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 64(4):639–653. https://doi.org/10.1016/j.eururo.2013.06.003
Dyrskjot L, Thykjaer T, Kruhoffer M, Jensen JL, Marcussen N, Hamilton-Dutoit S, Wolf H, Orntoft TF (2003) Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet 33(1):90–96. https://doi.org/10.1038/ng1061
Park J, Song C, Hong JH, Park BH, Cho YM, Kim CS, Ahn H (2009) Prognostic significance of non-papillary tumor morphology as a predictor of cancer progression and survival in patients with primary T1G3 bladder cancer. World J Urol 27(2):277–283. https://doi.org/10.1007/s00345-008-0350-4
Kulkarni GS, Finelli A, Fleshner NE, Jewett MA, Lopushinsky SR, Alibhai SM (2007) Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med 4(9):e284. https://doi.org/10.1371/journal.pmed.0040284
van der Meijden AP (1995) Practical approaches to the prevention and treatment of adverse reactions to BCG. Eur Urol 27(Suppl 1):23–28. https://doi.org/10.1159/000475205
Gonzalez-Padilla DA, Gonzalez-Diaz A, Guerrero-Ramos F, Rodriguez-Serrano A, Garcia-Jarabo E, Corona-laPuerta M, Rodriguez-Antolin A, Villacampa-Auba F (2021) Quality of life and adverse events in patients with nonmuscle invasive bladder cancer receiving adjuvant treatment with BCG, MMC, or chemohyperthermia. Urol Oncol 39(1):76e79–76e14. https://doi.org/10.1016/j.urolonc.2020.07.003
Thomsen JA, Nielsen Dominiak H, Lindgren MS, Jensen JB (2021) Adverse events of hyperthermic intravesical chemotherapy for non-muscle invasive bladder cancer patients. Scand J Urol 55(4):281–286. https://doi.org/10.1080/21681805.2021.1938664
Wei L, Li Q, Liang H, Jianbo L (2014) The quality of life in patients during intravesical treatment and correlation with local symptoms. J Chemother 26(3):165–168. https://doi.org/10.1179/1973947813Y.0000000126
Tan WS, Kelly JD (2018) Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol 15(11):667–685. https://doi.org/10.1038/s41585-018-0092-z
Racioppi M, Di Gianfrancesco L, Ragonese M, Palermo G, Sacco E, Bassi PF (2018) ElectroMotive drug administration (EMDA) of mitomycin C as first-line salvage therapy in high risk “BCG failure” non muscle invasive bladder cancer: 3 years follow-up outcomes. BMC Cancer 18(1):1224. https://doi.org/10.1186/s12885-018-5134-7
Juvet T, Mari A, Lajkosz K, Wallis CJ, Kuk C, Erlich A, Krimus L, Fleshner NE, Kulkarni GS, Zlotta AR (2020) Sequential administration of bacillus Calmette–Guerin (BCG) and electromotive drug administration (EMDA) of mitomycin C (MMC) for the treatment of high-grade nonmuscle invasive bladder cancer after BCG failure. Urol Oncol 38(11):850e859–850e815. https://doi.org/10.1016/j.urolonc.2020.06.031
Sanz Gomez I, Huguet J, Bravo A, Robalino J, Rodriguez Faba O, Territo A, Gaya JM, Palou J, Breda A (2023) Sequential treatment with bacillus Calmette–Guerin (BCG) and mitomycin C administered with electromotive drug administration (EMDA) in patients with high-risk nonmuscle invasive bladder cancer after BCG failure. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2023.03.002
Melgarejo Segura MT, Morales Martinez A, Yanez Castillo Y, Arrabal Polo MA, Gomez Lechuga P, Pareja Vilchez M, Arrabal Martin M (2023) Conductive hyperthermic chemotherapy versus electromotive drug administration of mitomycin C as intravesical adjuvant treatment of patients with intermediate or high-risk non-muscle invasive bladder cancer. Urol Oncol 41(2):109e101–109e108. https://doi.org/10.1016/j.urolonc.2022.10.019
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number: 82160148), and the “Cuiying Science and Technology Innovation” Program of the Second Hospital of Lanzhou University (Grant Number: CY2022-QN-B04).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Zhilong Dong. Analyzed the data: Chengyu You, Qingchao Li and Liangliang Qing. Contributed reagents/materials/analysis: Rongxin Li, Yanan Wang, and Long Cheng. Wrote the manuscript: Chengyu You and Qingchao Li. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
You, C., Li, Q., Qing, L. et al. Device-assisted intravesical chemotherapy versus bacillus Calmette–Guerin for intermediate or high-risk non-muscle invasive bladder cancer: a systematic reviewer and meta-analysis. Int Urol Nephrol 56, 103–120 (2024). https://doi.org/10.1007/s11255-023-03765-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03765-0