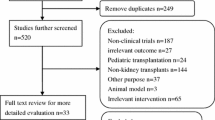
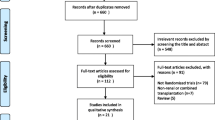
Abstract
In our study, we examined the efficacy of mTOR (mammalian target of rapamycin) inhibitors, specifically rapamycin (Rap), compared to calcineurin inhibitors (CNIs) in kidney transplantation. By conducting a comprehensive search across reputable databases (EMBASE, Scopus, PubMed, Cochrane, and Crossref), we gathered data for a six-month post-transplantation period. Our analysis revealed that mTOR inhibitor administration resulted in improved glomerular filtration rate (GFR) and serum creatinine levels. However, it is important to note that the mTOR inhibitor group had a higher incidence of acute rejection after biopsy. Through molecular modeling, we observed that Rap exhibited a superior binding affinity for mTOR compared to CNIs’ binding to calcineurin, probably contributing to the transplant rejection. Our meta-analysis supports the cautious use of an optimal mTOR inhibitor in conjunction with careful consideration of clinical features when minimizing CNIs early in the transplantation process. This is because mTOR inhibitors have complementary mechanisms of action, a low nephrotoxicity profile, and favorable outcomes in serum creatinine and GFR, which contribute to improved transplant survival.







Similar content being viewed by others
References
Salvadori M, Bertoni E (2013) Is it time to give up with calcineurin inhibitors in kidney transplantation? World J Transplant 3:7
Knops N, Levtchenko E, van den Heuvel B, Kuypers D (2013) From gut to kidney: transporting and metabolizing calcineurin-inhibitors in solid organ transplantation. Int J Pharm 452:14–35
Kumar J, Bridson JM, Sharma A, Halawa A (2017) Systematic review on role of mammalian target of rapamycin inhibitors as an alternative to calcineurin inhibitors in renal transplant: challenges and window to excel. Exp Clin Transplant 15:241–252
Hernández D, Hernández D, Martínez D, Martínez D, Gutiérrez E, Gutiérrez E et al (2011) Clinical evidence on the use of anti-mTOR drugs in renal transplantation. Nefrología (English Edition) 31:27–34
Li C, Yang CW (2009) The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol 5:513–519
Flechner SM (2009) Sirolimus in kidney transplantation indications and practical guidelines: de novo sirolimus-based therapy without calcineurin inhibitors. Transplantation 87:S1–S6
Lodhi S, Lamb K, Meier-Kriesche HU (2011) Improving long-term outcomes for transplant patients: Making the case for long-term disease-specific and multidisciplinary research. Am J Transplant 11:2264–2265
Gullestad L, Iversen M, Mortensen S-A, Eiskjær H, Riise GC, Mared L et al (2010) Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation 89:864–872
Serre J-E, Michonneau D, Bachy E, Noël L-H, Dubois V, Suberbielle C et al (2014) Maintaining calcineurin inhibition after the diagnosis of post-transplant lymphoproliferative disorder improves renal graft survival. Kidney Int 85:182–190
Gonzalez-Vilchez F, de Prada JV, Paniagua M, Gomez-Bueno M, Arizon J, Almenar L et al (2014) Use of mTOR inhibitors in chronic heart transplant recipients with renal failure: calcineurin-inhibitors conversion or minimization? Int J Cardiol 171:15–23
Zaza G, Tomei P, Ria P, Granata S, Boschiero L, Lupo A (2013) Systemic and nonrenal adverse effects occurring in renal transplant patients treated with mTOR inhibitors. Clin Dev Immunol 2013:1–13
Peddi VR, Wiseman A, Chavin K, Slakey D (2013) Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev 27:97–107
Rostaing L, Kamar N (2010) mTOR inhibitor/proliferation signal inhibitors: entering or leaving the field? J Nephrol 23:133–142
Ganschow R, Pape L, Sturm E, Bauer J, Melter M, Gerner P et al (2013) Growing experience with m TOR inhibitors in pediatric solid organ transplantation. Pediatr Transplant 17:694–706
Weir MR, Mulgaonkar S, Chan L, Shidban H, Waid TH, Preston D et al (2011) Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial. Kidney Int 79:897–907
Mjörnstedt L, Sørensen SS, von Zur Mühlen B, Jespersen B, Hansen J, Bistrup C et al (2012) Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant 12:2744–2753
Heilman RL, Younan K, Wadei HM, Mai ML, Reddy KS, Chakkera HA et al (2011) Results of a prospective randomized trial of sirolimus conversion in kidney transplant recipients on early corticosteroid withdrawal. Transplantation 92:767–773
Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U et al (2011) Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 377:837–847
Guba M, Pratschke J, Hugo C, Krämer BK, Nohr-Westphal C, Brockmann J et al (2010) Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation 90:175–183
Cibrik D, Silva HT Jr, Vathsala A, Lackova E, Cornu-Artis C, Walker RG et al (2013) Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 95:933–942
Pascual J, Berger SP, Witzke O, Tedesco H, Mulgaonkar S, Qazi Y et al (2018) Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol 29:1979–1991
Hoy MJ et al (2022) Structure-guided synthesis of FK506 and FK520 analogs with increased selectivity exhibit in vivo therapeutic efficacy against cryptococcus. MBio 13(3):e0104922
Lee YR et al (2005) Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood 105(10):3951–3955
Shityakov S et al (2017) In silico investigation of propofol binding sites in human serum albumin using explicit and implicit solvation models. Comput Biol Chem 70:191–197
Shityakov S et al (2020) Novel approach for characterizing propofol binding affinities to serum albumins from different species. ACS Omega 5(40):25543–25551
Sarukhanyan E et al (2019) Rational drug design of Axl tyrosine kinase type i inhibitors as promising candidates against cancer. Front Chem 7:920
Shityakov S et al (2021) Scaffold searching of FDA and EMA-approved drugs identifies lead candidates for drug repurposing in Alzheimer’s disease. Front Chem 9:736509
Case DA, Cheatham TE, Darden T, Gohlke H, Luo R, Merz KM, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26:1668–1688
Galvelis R et al (2019) A scalable molecular force field parameterization method based on density functional theory and quantum-level machine learning. J Chem Inf Model 59(8):3485–3493
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577–8593
Miyamoto S, Kollman PA (1992) Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem 13:952–962
Kollman PA, Massova I, Reyes C, Kuhn B, Huo S, Chong L et al (2000) Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res 33:889–897
Diekmann F, Campistol JM (2015) Practical considerations for the use of mTOR inhibitors. Transplant Res 4(Suppl 1):5
Sutherland AI, Akhtar MZ, Zilvetti M, Brockmann J, Ruse S, Fuggle SV et al (2014) Alemtuzumab and sirolimus in renal transplantation: six-year results of a single-arm prospective pilot study. Am J Transpl 14(3):677–684
Legaz I et al (2021) PCR array technology in biopsy samples identifies up-regulated mTOR Pathway genes as potential rejection biomarkers after kidney transplantation. Front Med (Lausanne) 8:547849
Gohlke H, Case DA (2004) Converging free energy estimates: MM-PB(GB)SA studies on the protein-protein complex Ras-Raf. J Comput Chem 25(2):238–250
Silva H Jr, Felipe CR, Garcia V, Neto E, Filho M, Contieri F et al (2013) Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant 13:3155–3163
Groetzner J, Kaczmarek I, Schulz U, Stegemann E, Kaiser K, Wittwer T et al (2009) Mycophenolate and sirolimus as calcineurin inhibitor-free immunosuppression improves renal function better than calcineurin inhibitor-reduction in late cardiac transplant recipients with chronic renal failure. Transplantation 87:726–733
Lebranchu Y, Thierry A, Toupance O, Westeel P, Etienne I, Thervet E et al (2009) Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 9:1115–1123
Zeng J et al (2021) Conversion from calcineurin inhibitors to mammalian target of rapamycin inhibitors in kidney transplant recipients: a systematic review and meta-analysis of randomized controlled trials. Front Immunol 12:663602
Lamas S (2005) Cellular mechanisms of vascular injury mediated by calcineurin inhibitors. Kidney Int 68:898
Novick AC et al (1989) Detrimental effect of cyclosporine on initial function of cadaver renal allografts following extended preservation. Results of a randomized prospective study. Transplantation 42:154
Kahan BD (1989) Cyclosporine. N Engl J Med 321:1725
Grenzi PC, Campos EF, Tedesco-Silva H Jr, Felipe CR, Soares MF, Medina-Pestana J et al (2018) Influence of immunosuppressive drugs on the CD30 molecule in kidney transplanted patients. Hum Immunol 79:550–557
Gatault P, Lebranchu Y (2013) Conversion to mTOR-inhibitor-based immunosuppression: which patients and when? Transplant Res 2:1–7
Kaczmarek I, Zaruba M-M, Beiras-Fernandez A, Reimann R, Nickel T, Grinninger C et al (2013) Tacrolimus with mycophenolate mofetil or sirolimus compared with calcineurin inhibitor-free immunosuppression (sirolimus/mycophenolate mofetil) after heart transplantation: 5-year results. J Heart Lung Transplant 32:277–284
Höcker B, Tönshoff B (2011) Calcineurin Inhibitor-Free Immunosuppression in Pediatric Renal Transplantation. Pediatr Drugs 13:49–69
Funding
This work received support from the Ministry of Science and Higher Education of the Russian Federation under goszadanie no. FSER-2021-0013. We would like to acknowledge the ITMO Fellowship and Professorship Program and Priority 2030 for their infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalid, H., Fareed, M.M., Dandekar, T. et al. Calcineurin and mTOR inhibitors in kidney transplantation: integrative metamodeling on transplant survival and kidney function. Int Urol Nephrol 56, 1403–1414 (2024). https://doi.org/10.1007/s11255-023-03754-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03754-3