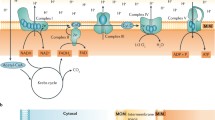
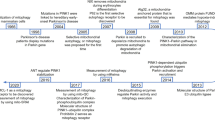
Abstract
As a high energy consumption organ, kidney relies on a large number of mitochondria to ensure normal physiological activities. Under specific stimulation, mitophagy and mitochondrial dynamics (fission, fusion) cooperatively regulate mitochondrial quality and participate in many life activities such as energy metabolism, inflammatory response, oxidative stress, cell senescence and death. Mitophagy plays a key role in the progression of acute kidney injury and chronic kidney disease. The early induction of oxidative stress in renal parenchyma, the activation of pro-inflammatory cytokines and TGF-β signal pathway are closely related to renal interstitial fibrosis. Macrophage reprogramming is also considered to be an important participant in the progression of kidney fibrosis. This review summarizes the molecular mechanism of mitochondrial autophagy and its relationship with the pathway of promoting fibrosis, and discusses the possibility of restoring mitophagy balance as a pharmacological target for the treatment of renal interstitial fibrosis, so as to provide new ideas for more efficient anti-fibrosis and delay the progress of chronic kidney disease.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Aguilera MO, Robledo E, Melani M, Wappner P, Colombo MI (2022) FKBP8 is a novel molecule that participates in the regulation of the autophagic pathway. Biochim Biophys Acta Mol Cell Res 1869(5):119212. https://doi.org/10.1016/j.bbamcr.2022.119212
Aparicio-Trejo OE, Tapia E, Molina-Jijon E, Medina-Campos ON, Macias-Ruvalcaba NA, Leon-Contreras JC et al (2017) Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: relation to oxidative stress and mitochondrial bioenergetics. BioFactors 43(2):293–310. https://doi.org/10.1002/biof.1338
Bai J, Liu F (2021) cGAS-STING signaling and function in metabolism and kidney diseases. J Mol Cell Biol 13(10):728–738. https://doi.org/10.1093/jmcb/mjab066
Ban T, Ishihara T, Kohno H, Saita S, Ichimura A, Maenaka K et al (2017) Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat Cell Biol 19(7):856–863. https://doi.org/10.1038/ncb3560
Bhatia D, Capili A, Nakahira K, Muthukumar T, Torres LK, Choi A et al (2022) Conditional deletion of myeloid-specific mitofusin 2 but not mitofusin 1 promotes kidney fibrosis. Kidney Int 101(5):963–986. https://doi.org/10.1016/j.kint.2022.01.030
Bhatia D, Chung KP, Nakahira K, Patino E, Rice MC, Torres LK et al (2019) Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight. https://doi.org/10.1172/jci.insight.132826
Bhujabal Z, Birgisdottir AB, Sjottem E, Brenne HB, Overvatn A, Habisov S et al (2017) FKBP8 recruits LC3A to mediate Parkin-independent mitophagy. EMBO Rep 18(6):947–961. https://doi.org/10.15252/embr.201643147
Boor P, Ostendorf T, Floege J (2014) PDGF and the progression of renal disease. Nephrol Dial Transplant 29(Suppl 1):i45–i54. https://doi.org/10.1093/ndt/gft273
Bratic A, Larsson NG (2013) The role of mitochondria in aging. J Clin Invest 123(3):951–957. https://doi.org/10.1172/JCI64125
Cai C, Wu F, Zhuang B, Ou Q, Peng X, Shi N et al (2022) Empagliflozin activates Wnt/beta-catenin to stimulate FUNDC1-dependent mitochondrial quality surveillance against type-3 cardiorenal syndrome. Mol Metab 64:101553. https://doi.org/10.1016/j.molmet.2022.101553
Chao H, Lin C, Zuo Q, Liu Y, Xiao M, Xu X et al (2019) Cardiolipin-dependent mitophagy guides outcome after traumatic brain injury. J Neurosci 39(10):1930–1943. https://doi.org/10.1523/JNEUROSCI.3415-17.2018
Chen M, Chen Z, Wang Y, Tan Z, Zhu C, Li Y et al (2016) Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 12(4):689–702. https://doi.org/10.1080/15548627.2016.1151580
Chen Y, Dorn GN (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340(6131):471–475. https://doi.org/10.1126/science.1231031
Choi ME (2020) Autophagy in kidney disease. Annu Rev Physiol 82:297–322. https://doi.org/10.1146/annurev-physiol-021119-034658
Chu CT, Bayir H, Kagan VE (2014) LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: implications for Parkinson disease. Autophagy 10(2):376–378. https://doi.org/10.4161/auto.27191
Chung KW, Dhillon P, Huang S, Sheng X, Shrestha R, Qiu C et al (2019) Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab 30(4):784–799. https://doi.org/10.1016/j.cmet.2019.08.003
Di Rita A, Peschiaroli A, D’Acunzo P, Strobbe D, Hu Z, Gruber J et al (2018) HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKalpha. Nat Commun 9(1):3755. https://doi.org/10.1038/s41467-018-05722-3
Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB et al (2010) Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 285(36):27879–27890. https://doi.org/10.1074/jbc.M110.119537
Ding Y, Kim S, Lee SY, Koo JK, Wang Z, Choi ME (2014) Autophagy regulates TGF-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol 25(12):2835–2846. https://doi.org/10.1681/ASN.2013101068
Duan P, Tan J, Miao Y, Zhang Q (2022) PINK1/Parkin-mediated mitophagy plays a protective role in albumin overload-induced renal tubular cell injury. Front Biosci (Landmark Ed) 27(6):184. https://doi.org/10.31083/j.fbl2706184
Dudek J (2017) Role of cardiolipin in mitochondrial signaling pathways. Front Cell Dev Biol 5:90. https://doi.org/10.3389/fcell.2017.00090
Fujimura R, Yamamoto T, Takabatake Y, Takahashi A, Namba-Hamano T, Minami S et al (2020) Autophagy protects kidney from phosphate-induced mitochondrial injury. Biochem Biophys Res Commun 524(3):636–642. https://doi.org/10.1016/j.bbrc.2020.01.137
Gao A, Jiang J, Xie F, Chen L (2020) Bnip3 in mitophagy: novel insights and potential therapeutic target for diseases of secondary mitochondrial dysfunction. Clin Chim Acta 506:72–83. https://doi.org/10.1016/j.cca.2020.02.024
Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R et al (2016) Mitochondrial diseases. Nat Rev Dis Primers 2:16080. https://doi.org/10.1038/nrdp.2016.80
Gottlieb RA, Piplani H, Sin J, Sawaged S, Hamid SM, Taylor DJ et al (2021) At the heart of mitochondrial quality control: many roads to the top. Cell Mol Life Sci 78(8):3791–3801. https://doi.org/10.1007/s00018-021-03772-3
Hahn A, Zuryn S (2019) Mitochondrial genome (mtDNA) mutations that generate reactive oxygen species. Antioxidants (Basel) 8(9):392. https://doi.org/10.3390/antiox8090392
He YL, Li J, Gong SH, Cheng X, Zhao M, Cao Y et al (2022) BNIP3 phosphorylation by JNK1/2 promotes mitophagy via enhancing its stability under hypoxia. Cell Death Dis 13(11):966. https://doi.org/10.1038/s41419-022-05418-z
Holmstrom KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15(6):411–421. https://doi.org/10.1038/nrm3801
Iorio R, Celenza G, Petricca S (2021) Mitophagy: molecular mechanisms, new concepts on Parkin activation and the emerging role of AMPK/ULK1 axis. Cells 11(1):30. https://doi.org/10.3390/cells11010030
Ismail T, Kim Y, Lee H, Lee DS, Lee HS (2019) Interplay between mitochondrial peroxiredoxins and ROS in cancer development and progression. Int J Mol Sci 20(18):4407. https://doi.org/10.3390/ijms20184407
Jadiya P, Tomar D (2020) Mitochondrial protein quality control mechanisms. Genes (Basel) 11(5):563. https://doi.org/10.3390/genes11050563
Jimenez-Uribe AP, Bellido B, Aparicio-Trejo OE, Tapia E, Sanchez-Lozada LG, Hernandez-Santos JA et al (2021) Temporal characterization of mitochondrial impairment in the unilateral ureteral obstruction model in rats. Free Radic Biol Med 172:358–371. https://doi.org/10.1016/j.freeradbiomed.2021.06.019
Jin L, Yu B, Liu G, Nie W, Wang J, Chen J et al (2022) Mitophagy induced by UMI-77 preserves mitochondrial fitness in renal tubular epithelial cells and alleviates renal fibrosis. FASEB J 36(6):e22342. https://doi.org/10.1096/fj.202200199RR
Jin SM, Youle RJ (2013) The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 9(11):1750–1757. https://doi.org/10.4161/auto.26122
Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C et al (2016) NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell Death Differ 23(7):1140–1151. https://doi.org/10.1038/cdd.2015.160
Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA et al (2014) Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J 33(23):2798–2813. https://doi.org/10.15252/embj.201488658
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119(6):1420–1428. https://doi.org/10.1172/JCI39104
Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W (2015) The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS ONE 10(12):e143961. https://doi.org/10.1371/journal.pone.0143961
Kim SM, Kim YG, Kim DJ, Park SH, Jeong KH, Lee YH et al (2018) Inflammasome-independent role of NLRP3 mediates mitochondrial regulation in renal injury. Front Immunol 9:2563. https://doi.org/10.3389/fimmu.2018.02563
Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten LH et al (2021) Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593(7859):435–439. https://doi.org/10.1038/s41586-021-03510-6
Kuang Y, Ma K, Zhou C, Ding P, Zhu Y, Chen Q et al (2016) Structural basis for the phosphorylation of FUNDC1 LIR as a molecular switch of mitophagy. Autophagy 12(12):2363–2373. https://doi.org/10.1080/15548627.2016.1238552
Landes T, Emorine LJ, Courilleau D, Rojo M, Belenguer P, Arnaune-Pelloquin L (2010) The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep 11(6):459–465. https://doi.org/10.1038/embor.2010.50
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL et al (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524(7565):309–314. https://doi.org/10.1038/nature14893
Lee SY, Kim SI, Choi ME (2015) Therapeutic targets for treating fibrotic kidney diseases. Transl Res 165(4):512–530. https://doi.org/10.1016/j.trsl.2014.07.010
Lee WC, Chiu CH, Chen JB, Chen CH, Chang HW (2016) Mitochondrial fission increases apoptosis and decreases autophagy in renal proximal tubular epithelial cells treated with high glucose. DNA Cell Biol 35(11):657–665. https://doi.org/10.1089/dna.2016.3261
Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ (2010) Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 177(3):1065–1071. https://doi.org/10.2353/ajpath.2010.090923
Li M, Jia J, Zhang X, Dai H (2020) Selective binding of mitophagy receptor protein Bcl-rambo to LC3/GABARAP family proteins. Biochem Biophys Res Commun 530(1):292–300. https://doi.org/10.1016/j.bbrc.2020.07.039
Li N, Wang H, Jiang C, Zhang M (2018) Renal ischemia/reperfusion-induced mitophagy protects against renal dysfunction via Drp1-dependent-pathway. Exp Cell Res 369(1):27–33. https://doi.org/10.1016/j.yexcr.2018.04.025
Li S, Lin Q, Shao X, Zhu X, Wu J, Wu B et al (2020) Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic Biol Med 152:632–649. https://doi.org/10.1016/j.freeradbiomed.2019.12.005
Lim GG, Lim KL (2017) Parkin-independent mitophagy-FKBP8 takes the stage. EMBO Rep 18(6):864–865. https://doi.org/10.15252/embr.201744313
Liu T, Yang Q, Zhang X, Qin R, Shan W, Zhang H et al (2020) Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci 257:118116. https://doi.org/10.1016/j.lfs.2020.118116
Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong W et al (2018) Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol 154:203–212. https://doi.org/10.1016/j.bcp.2018.05.007
Lu YP, Wu HW, Zhu T, Li XT, Zuo J, Hasan AA et al (2022) Empagliflozin reduces kidney fibrosis and improves kidney function by alternative macrophage activation in rats with 5/6-nephrectomy. Biomed Pharmacother 156:113947. https://doi.org/10.1016/j.biopha.2022.113947
Luevano-Martinez LA, Pinto I, Yoshinaga MY, Miyamoto S (2022) In yeast, cardiolipin unsaturation level plays a key role in mitochondrial function and inner membrane integrity. Biochim Biophys Acta Bioenerg 1863(7):148587. https://doi.org/10.1016/j.bbabio.2022.148587
Lv M, Wang C, Li F, Peng J, Wen B, Gong Q et al (2017) Structural insights into the recognition of phosphorylated FUNDC1 by LC3B in mitophagy. Protein Cell 8(1):25–38. https://doi.org/10.1007/s13238-016-0328-8
Lv W, Booz GW, Wang Y, Fan F, Roman RJ (2018) Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol 820:65–76. https://doi.org/10.1016/j.ejphar.2017.12.016
Matsuda N (2016) Phospho-ubiquitin: upending the PINK-Parkin-ubiquitin cascade. J Biochem 159(4):379–385. https://doi.org/10.1093/jb/mvv125
Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Lower B, Wunderlich FT et al (2008) Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 22(4):476–488. https://doi.org/10.1101/gad.460708
Murakawa T, Okamoto K, Omiya S, Taneike M, Yamaguchi O, Otsu K (2019) A mammalian mitophagy receptor, Bcl2-L-13, recruits the ULK1 complex to induce mitophagy. Cell Rep 26(2):338–345. https://doi.org/10.1016/j.celrep.2018.12.050
Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T et al (2015) Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun 6:7527. https://doi.org/10.1038/ncomms8527
Mylonas KJ, O’Sullivan ED, Humphries D, Baird DP, Docherty MH, Neely SA et al (2021) Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abb0203
Naik E, Dixit VM (2011) Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 208(3):417–420. https://doi.org/10.1084/jem.20110367
Ni Y, Wu GH, Cai JJ, Zhang R, Zheng Y, Liu JQ et al (2022) Tubule-mitophagic secretion of SerpinG1 reprograms macrophages to instruct anti-septic acute kidney injury efficacy of high-dose ascorbate mediated by NRF2 transactivation. Int J Biol Sci 18(13):5168–5184. https://doi.org/10.7150/ijbs.74430
Nikolic-Paterson DJ, Wang S, Lan HY (2014) Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl (2011) 4(1):34–38. https://doi.org/10.1038/kisup.2014.7
Otera H, Miyata N, Kuge O, Mihara K (2016) Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J Cell Biol 212(5):531–544. https://doi.org/10.1083/jcb.201508099
Palikaras K, Lionaki E, Tavernarakis N (2016) Mitophagy: in sickness and in health. Mol Cell Oncol 3(1):e1056332. https://doi.org/10.1080/23723556.2015.1056332
Perry HM, Huang L, Wilson RJ, Bajwa A, Sesaki H, Yan Z et al (2018) Dynamin-related protein 1 deficiency promotes recovery from AKI. J Am Soc Nephrol 29(1):194–206. https://doi.org/10.1681/ASN.2017060659
Pfanner N, Warscheid B, Wiedemann N (2019) Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol 20(5):267–284. https://doi.org/10.1038/s41580-018-0092-0
Pimentel JJ, Montero A, Wang S, Yosipiv I, El-Dahr S, Martinez-Maldonado M (1995) Sequential changes in renal expression of renin-angiotensin system genes in acute unilateral ureteral obstruction. Kidney Int 48(4):1247–1253. https://doi.org/10.1038/ki.1995.408
Rogov VV, Suzuki H, Marinkovic M, Lang V, Kato R, Kawasaki M et al (2017) Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins. Sci Rep 7(1):1131. https://doi.org/10.1038/s41598-017-01258-6
Savio-Silva C, Soinski-Sousa PE, Simplicio-Filho A, Bastos R, Beyerstedt S, Rangel EB (2021) Therapeutic potential of mesenchymal stem cells in a pre-clinical model of diabetic kidney disease and obesity. Int J Mol Sci 22(4):1546. https://doi.org/10.3390/ijms22041546
Schuster R, Okamoto K (2022) An overview of the molecular mechanisms of mitophagy in yeast. Biochim Biophys Acta Gen Subj 1866(11):130203. https://doi.org/10.1016/j.bbagen.2022.130203
See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR et al (2019) Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 95(1):160–172. https://doi.org/10.1016/j.kint.2018.08.036
Strappazzon F, Nazio F, Corrado M, Cianfanelli V, Romagnoli A, Fimia GM et al (2015) AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ 22(3):419–432. https://doi.org/10.1038/cdd.2014.139
Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM (2017) Cellular senescence in renal ageing and disease. Nat Rev Nephrol 13(2):77–89. https://doi.org/10.1038/nrneph.2016.183
Sun D, Wu R, Zheng J, Li P, Yu L (2018) Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res 28(4):405–415. https://doi.org/10.1038/s41422-018-0017-7
Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V et al (2017) Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1beta and IL-18 and arrests CKD. J Am Soc Nephrol 28(5):1437–1449. https://doi.org/10.1681/ASN.2016070761
Tang C, Han H, Yan M, Zhu S, Liu J, Liu Z et al (2018) PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14(5):880–897. https://doi.org/10.1080/15548627.2017.1405880
Tang H, Yang M, Liu Y, Zhu X, Liu S, Liu H et al (2022) Melatonin alleviates renal injury by activating mitophagy in diabetic nephropathy. Front Endocrinol (Lausanne) 13:889729. https://doi.org/10.3389/fendo.2022.889729
Tang PM, Nikolic-Paterson DJ, Lan HY (2019) Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol 15(3):144–158. https://doi.org/10.1038/s41581-019-0110-2
Tang PM, Zhou S, Li CJ, Liao J, Xiao J, Wang QM et al (2018) The proto-oncogene tyrosine protein kinase Src is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int 93(1):173–187. https://doi.org/10.1016/j.kint.2017.07.026
Tilokani L, Nagashima S, Paupe V, Prudent J (2018) Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62(3):341–360. https://doi.org/10.1042/EBC20170104
Toki D, Zhang W, Hor KL, Liuwantara D, Alexander SI, Yi Z et al (2014) The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am J Transplant 14(9):2126–2136. https://doi.org/10.1111/ajt.12803
Topcu SO, Celik S, Erturhan S, Erbagci A, Yagci F, Ucak R (2008) Verapamil prevents the apoptotic and hemodynamic changes in response to unilateral ureteral obstruction. Int J Urol 15(4):350–355. https://doi.org/10.1111/j.1442-2042.2008.01992.x
Turco E, Savova A, Gere F, Ferrari L, Romanov J, Schuschnig M et al (2021) Reconstitution defines the roles of p62, NBR1 and TAX1BP1 in ubiquitin condensate formation and autophagy initiation. Nat Commun 12(1):5212. https://doi.org/10.1038/s41467-021-25572-w
Vargas J, Wang C, Bunker E, Hao L, Maric D, Schiavo G et al (2019) Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol Cell 74(2):347–362. https://doi.org/10.1016/j.molcel.2019.02.010
Wang D, Kang L, Chen C, Guo J, Du L, Zhou D et al (2022) Loss of legumain induces premature senescence and mediates aging-related renal fibrosis. Aging Cell 21(3):e13574. https://doi.org/10.1111/acel.13574
Wang J, Zhu P, Li R, Ren J, Zhang Y, Zhou H (2020) Bax inhibitor 1 preserves mitochondrial homeostasis in acute kidney injury through promoting mitochondrial retention of PHB2. Theranostics 10(1):384–397. https://doi.org/10.7150/thno.40098
Wang J, Zhu P, Li R, Ren J, Zhou H (2020) Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol 30:101415. https://doi.org/10.1016/j.redox.2019.101415
Wang S, Meng XM, Ng YY, Ma FY, Zhou S, Zhang Y et al (2016) TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 7(8):8809–8822. https://doi.org/10.18632/oncotarget.6604
Wei Y, Chiang WC, Sumpter RJ, Mishra P, Levine B (2017) Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 168(1–2):224–238. https://doi.org/10.1016/j.cell.2016.11.042
Wild P, McEwan DG, Dikic I (2014) The LC3 interactome at a glance. J Cell Sci 127(Pt 1):3–9. https://doi.org/10.1242/jcs.140426
Wu J, Raman A, Coffey NJ, Sheng X, Wahba J, Seasock MJ et al (2021) The key role of NLRP3 and STING in APOL1-associated podocytopathy. J Clin Investig. https://doi.org/10.1172/JCI136329
Wu WH, Zhang MP, Zhang F, Liu F, Hu ZX, Hu QD et al (2011) The role of programmed cell death in streptozotocin-induced early diabetic nephropathy. J Endocrinol Invest 34(9):e296–e301. https://doi.org/10.3275/7741
Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H et al (2022) Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 55(8):1370–1385. https://doi.org/10.1016/j.immuni.2022.06.007
Xiao JJ, Liu Q, Li Y, Peng FF, Wang S, Zhang Z et al (2022) Regulator of calcineurin 1 deletion attenuates mitochondrial dysfunction and apoptosis in acute kidney injury through JNK/Mff signaling pathway. Cell Death Dis 13(9):774. https://doi.org/10.1038/s41419-022-05220-x
Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D et al (2017) The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11:297–311. https://doi.org/10.1016/j.redox.2016.12.022
Xiao T, Guan X, Nie L, Wang S, Sun L, He T et al (2014) Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol Cell Biochem 394(1–2):145–154. https://doi.org/10.1007/s11010-014-2090-7
Xie Y, Liu J, Kang R, Tang D (2020) Mitophagy receptors in tumor biology. Front Cell Dev Biol 8:594203. https://doi.org/10.3389/fcell.2020.594203
Xiong W, Ma Z, An D, Liu Z, Cai W, Bai Y et al (2019) Mitofusin 2 participates in mitophagy and mitochondrial fusion against angiotensin ii-induced cardiomyocyte injury. Front Physiol 10:411. https://doi.org/10.3389/fphys.2019.00411
Yamashita SI, Kanki T (2017) How autophagy eats large mitochondria: autophagosome formation coupled with mitochondrial fragmentation. Autophagy 13(5):980–981. https://doi.org/10.1080/15548627.2017.1291113
Yan C, Gong L, Chen L, Xu M, Abou-Hamdan H, Tang M et al (2020) PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy 16(3):419–434. https://doi.org/10.1080/15548627.2019.1628520
Yang X, Yin H, Zhang Y, Li X, Tong H, Zeng Y et al (2018) Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1alpha activation. Int J Oncol 53(1):215–224. https://doi.org/10.3892/ijo.2018.4376
Yoo SM, Yamashita SI, Kim H, Na D, Lee H, Kim SJ et al (2020) FKBP8 LIRL-dependent mitochondrial fragmentation facilitates mitophagy under stress conditions. FASEB J 34(2):2944–2957. https://doi.org/10.1096/fj.201901735R
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12(1):9–14. https://doi.org/10.1038/nrm3028
Youle RJ, van der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337(6098):1062–1065. https://doi.org/10.1126/science.1219855
Yuan Y, Zheng Y, Zhang X, Chen Y, Wu X, Wu J et al (2017) BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13(10):1754–1766. https://doi.org/10.1080/15548627.2017.1357792
Zeng LF, Xiao Y, Sun L (2019) A glimpse of the mechanisms related to renal fibrosis in diabetic nephropathy. Adv Exp Med Biol 1165:49–79. https://doi.org/10.1007/978-981-13-8871-2_4
Zhang Y, Wang Y, Xu J, Tian F, Hu S, Chen Y et al (2019) Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res 66(2):e12542. https://doi.org/10.1111/jpi.12542
Zhao Y, Guo Y, Jiang Y, Zhu X, Liu Y, Zhang X (2017) Mitophagy regulates macrophage phenotype in diabetic nephropathy rats. Biochem Biophys Res Commun 494(1–2):42–50. https://doi.org/10.1016/j.bbrc.2017.10.088
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ 25(6):1080–1093. https://doi.org/10.1038/s41418-018-0086-7
Funding
This work received support by the Joint Fund for Innovation and Development of Natural Science Foundation of Shandong Province, China (ZR2022LZY005).
Author information
Authors and Affiliations
Contributions
Z-AG and Y-YL conceived the study. JS and CL wrote and revised the manuscript. Y-YL revised the manuscript. All the authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, J., Liu, C., Liu, YY. et al. Mitophagy in renal interstitial fibrosis. Int Urol Nephrol 56, 167–179 (2024). https://doi.org/10.1007/s11255-023-03686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03686-y