Abstract
Purpose
To develop an assistant tool based on machine learning for early frailty screening in patients receiving maintenance hemodialysis.
Methods
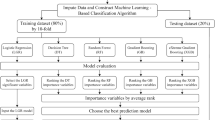
This is a single-center retrospective study. 141 participants’ basic information, scale results and laboratory findings were collected and the FRAIL scale was used to assess frailty. Then participants were divided into the frailty group (n = 84) and control group (n = 57). After feature selection, data split and oversampling, ten commonly used binary machine learning methods were performed and a voting classifier was developed.
Results
The grade results of Clinical Frailty Scale, age, serum magnesium, lactate dehydrogenase, comorbidity and fast blood glucose were considered to be the best feature set for early frailty screening. After abandoning models with overfitting or poor performance, the voting classifier based on Support Vector Machine, Adaptive Boosting and Naive Bayes achieved a good screening performance (sensitivity: 68.24% ± 8.40%, specificity:72.50% ± 11.81%, F1 score: 72.55% ± 4.65%, AUC:78.38% ± 6.94%).
Conclusion
A simple and efficient early frailty screening assistant tool for patients receiving maintenance hemodialysis based on machine learning was developed. It can provide assistance on frailty, especially pre-frailty screening and decision-making tasks.



Similar content being viewed by others
Data availability
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bikbov B et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733. https://doi.org/10.1016/s0140-6736(20)30045-3
Nixon AC et al (2018) Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J 11(2):236–245. https://doi.org/10.1093/ckj/sfx134
Kojima G, Liljas AEM, Iliffe S (2019) Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy 12:23–30. https://doi.org/10.2147/rmhp.S168750
Yoneki K et al (2019) Association between frailty and bone loss in patients undergoing maintenance hemodialysis. J Bone Miner Metab 37(1):81–89. https://doi.org/10.1007/s00774-017-0898-4
Lorenz EC et al (2021) Frailty in CKD and transplantation. Kidney International Reports 6(9):2270–2280. https://doi.org/10.1016/j.ekir.2021.05.025
Fitzpatrick J et al (2019) Frailty, body composition and the risk of mortality in incident hemodialysis patients: the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease study. Nephrol Dial Transplant 34(2):346–354. https://doi.org/10.1093/ndt/gfy124
Jiang X et al (2020) In-hospital outcomes of patients on maintenance dialysis with frailty: 10-year results from the US national inpatient sample database. J Ren Nutr 30(6):526–534. https://doi.org/10.1053/j.jrn.2019.12.007
Zhao Y, Liu Q, Ji J (2020) The prevalence of frailty in patients on hemodialysis: a systematic review and meta-analysis. Int Urol Nephrol 52(1):115–120. https://doi.org/10.1007/s11255-019-02310-2
Sezgin D et al (2020) Pre-frailty as a multi-dimensional construct: a systematic review of definitions in the scientific literature. Geriatr Nurs 41(2):139–146. https://doi.org/10.1016/j.gerinurse.2019.08.004
Dent E, Kowal P, Hoogendijk EO (2016) Frailty measurement in research and clinical practice: a review. Eur J Intern Med 31:3–10. https://doi.org/10.1016/j.ejim.2016.03.007
Worthen G, Tennankore K (2019) Frailty screening in chronic kidney disease: current perspectives. Int J Nephrol Renov Dis 12:229–239. https://doi.org/10.2147/ijnrd.S228956
Shah SM, Khan RA (2020) Secondary use of electronic health record: opportunities and challenges. Ieee Access 8:136947–136965. https://doi.org/10.1109/access.2020.3011099
Philipp Hassler A et al (2019) Importance of medical data preprocessing in predictive modeling and risk factor discovery for the frailty syndrome. Bmc Med Inform Decis Mak. https://doi.org/10.1186/s12911-019-0747-6
Handelman GS et al (2018) eDoctor: machine learning and the future of medicine. J Intern Med 284(6):603–619. https://doi.org/10.1111/joim.12822
Aponte-Hao S et al (2021) Machine learning for identification of frailty in Canadian primary care practices. Int J Popul Data Sci (Ijpds). https://doi.org/10.23889/ijpds.v6i1.1650
Ambagtsheer RC et al (2020) The application of artificial intelligence (AI) techniques to identify frailty within a residential aged care administrative data set. Int J Med Inform. https://doi.org/10.1016/j.ijmedinf.2020.104094
Woo J et al (2018) Variability in repeated blood pressure measurements as a marker of frailty. J Nutr Health Aging 22(9):1122–1127. https://doi.org/10.1007/s12603-018-1082-9
Park C et al (2021) Digital biomarker representing frailty phenotypes: the use of machine learning and sensor-based sit-to-stand test. Sensors. https://doi.org/10.3390/s21093258
Minici D et al (2022) Towards automated assessment of frailty status using a wrist-worn device. IEEE J Biomed Health Inform 26(3):1013–1022. https://doi.org/10.1109/jbhi.2021.3100979
Morley JE, Malmstrom TK, Miller DK (2012) A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 16(7):601–608. https://doi.org/10.1007/s12603-012-0084-2
Chao C-T et al (2015) Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology 20(5):321–328. https://doi.org/10.1111/nep.12401
Yuan H et al (2020) Exploring psychosocial factors associated with frailty incidence among patients undergoing maintenance hemodialysis. J Clin Nurs 29(9–10):1695–1703. https://doi.org/10.1111/jocn.15225
Cruz-Jentoft AJ et al (2017) Nutrition, frailty, and sarcopenia. Aging Clin Exp Res 29(1):43–48. https://doi.org/10.1007/s40520-016-0709-0
Poli S et al (2017) Frailty is associated with socioeconomic and lifestyle factors in community-dwelling older subjects. Aging Clin Exp Res 29(4):721–728. https://doi.org/10.1007/s40520-016-0623-5
Drew DA et al (2017) Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis 69(6):780–787. https://doi.org/10.1053/j.ajkd.2016.11.015
Breiman L (2001) Random forests. Mach Learn 45(1):5–32. https://doi.org/10.1023/a:1010933404324
Althnian A et al (2021) Impact of dataset size on classification performance: an empirical evaluation in the medical domain. Appl Sci-Basel. https://doi.org/10.3390/app11020796
Chawla NV et al (2002) SMOTE: synthetic minority over-sampling technique. J Artif Intell Res 16:321–357. https://doi.org/10.1613/jair.953
Sokolova M, Lapalme G (2009) A systematic analysis of performance measures for classification tasks. Inf Process Manag 45(4):427–437. https://doi.org/10.1016/j.ipm.2009.03.002
Rizwan A et al (2021) Enhanced optimization-based voting classifier and chained multi-objective regressor for effective groundwater resource management. Ieee Access 9:168329–168341. https://doi.org/10.1109/access.2021.3133889
Rodriguez-Manas L et al (2020) ICFSR task force perspective on biomarkers for sarcopenia and frailty. J Frailty Aging 9(1):4–8. https://doi.org/10.14283/jfa.2019.32
Lo Piano F, Corsonello A, Corica F (2019) Magnesium and elderly patient: the explored paths and the ones to be explored: a review. Magnes Res 32(1):1–15. https://doi.org/10.1684/mrh.2019.0453
Chen L-H, Wu L-W (2021) Association between serum lactate dehydrogenase and frailty among individuals with metabolic syndrome. PLoS ONE. https://doi.org/10.1371/journal.pone.0256315
Fung E et al (2021) Characterising frailty, metrics of continuous glucose monitoring, and mortality hazards in older adults with type 2 diabetes on insulin therapy (HARE): a prospective, observational cohort study. Lancet Healthy Longev 2(11):E724–E735 (<Go to ISI>://WOS:000717480300011)
Chung SM et al (2021) Daytime glycemic variability and frailty in older patients with diabetes: a pilot study using continuous glucose monitoring. J Korean Med Sci. https://doi.org/10.3346/jkms.2021.36.e190
Holzinger A et al (2019) Interactive machine learning: experimental evidence for the human in the algorithmic loop: a case study on ant colony optimization. Appl Intell 49(7):2401–2414. https://doi.org/10.1007/s10489-018-1361-5
Rohani A, Mamarabadi M (2019) Free alignment classification of Dikarya fungi using some machine learning methods. Neural Comput Appl 31(11):6995–7016. https://doi.org/10.1007/s00521-018-3539-5
Lundberg SM, Lee S-I (2017) A unified approach to interpreting model predictions. In: 31st Annual Conference on neural information processing systems (NIPS), 2017, Long Beach, CA
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFC2005600), West China Nursing Discipline Development Special Fund Project, Sichuan University (HXHL21015), Major Research Programs of Science & Technology Department of Sichuan Province (2022ZDZX0032) and Project of Sichuan Luzhou Science and Technology Bureau (2022CDLZ-25).
Author information
Authors and Affiliations
Contributions
HY and YC were the principal leaders. They conceived this study, participated in design and coordination. WL and HL participated in design and coordination, helped to draft manuscript. Data were analysed by HL and XW. SY, YP, and XL searched literatures, collected the data of patients receiving maintenance hemodialysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. This study received approval from the Ethics Committee of Sichuan University (ethical approval number: 2020[1002]) and was performed in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived by the Ethics Committee of Sichuan University because this was an observational, cross-sectional study and patients’ identifying information had been removed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, W., Liao, H., Wang, X. et al. A machine learning-based assistant tool for early frailty screening of patients receiving maintenance hemodialysis. Int Urol Nephrol 56, 223–235 (2024). https://doi.org/10.1007/s11255-023-03640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03640-y