Abstract
Chronic kidney disease is among the most common causes of mortality and morbidity in adult population with limited therapeutic approaches including various medications and kidney replacement therapies. Kidney transplantation is the gold standard therapeutic alternative for the management of chronic kidney disease; nonetheless, important drawbacks include the lack of adequate living or deceased donors, high rates of pre- and post-operative complications including surgical complications, infectious complications and medication-induced adverse effects. With the latest preclinical and in vitro studies demonstrating the potentiality of kidney cells obtained from diseased kidneys to convert into fully functional kidney cells lead to a novel therapeutic alternative referred as autologous selected renal cell transplantation. Even though the clinical studies investigating the efficiency and adverse effects of autologous selected renal cell transplantation are limited, it is no doubt promising. The need for future large-scale studies on chronic kidney disease patients from a diversity of etiologies is clear for the better establishment of the therapeutic potential of autologous selected renal cell transplantation. In this narrative review, our aim is to evaluate the role of renal autologous stem cell therapy in the management of chronic kidney disease.


Similar content being viewed by others
Data availability
Our manuscript has no associated data.
References
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS et al (2016) Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE 11(7):e0158765
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, Adebayo OM, Afarideh M, Agarwal SK, Agudelo-Botero M, Ahmadian E (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global burden of disease study 2017. Lancet 395(10225):709–733
Chen TK, Knicely DH, Grams ME (2019) Chronic kidney disease diagnosis and management: a review. JAMA 322(13):1294–1304
Hannan M, Ansari S, Meza N, Anderson AH, Srivastava A, Waikar S et al (2021) Risk factors for CKD progression: overview of findings from the CRIC study. Clin J Am Soc Nephrol 16(4):648–659
Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR (2020) Targeting the progression of chronic kidney disease. Nat Rev Nephrol 16(5):269–288
Haberal M, Boyvat F, Akdur A, Kırnap M, Özçelik Ü, Yarbuğ KF (2016) Surgical complications after kidney transplantation. Exp Clin Transplant 14(6):587–595
Agrawal A, Ison MG, Danziger-Isakov L (2022) Long-term infectious complications of kidney transplantation. Clin J Am Soc Nephrol 17(2):286–295
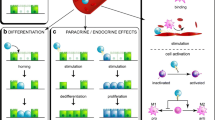
Witzgall R, Brown D, Schwarz C, Bonventre JV (1994) Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93(5):2175–2188
Lin F, Moran A, Igarashi P (2005) Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115(7):1756–1764
Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F et al (2006) Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17(9):2443–2456
Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS et al (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2(3):284–291
Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV (2011) Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 108(22):9226–9231
Kramann R, Humphreys BD (2014) Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol 34(4):374–383
Koniusz S, Andrzejewska A, Muraca M, Srivastava AK, Janowski M, Lukomska B (2016) Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci 10:109
Yu B, Zhang X, Li X (2014) Exosomes derived from mesenchymal stem cells. Int J Mol Sci 15(3):4142–4157
Lai RC, Chen TS, Lim SK (2011) Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med 6(4):481–492
Lindoso RS, Collino F, Bruno S, Araujo DS, Sant’Anna JF, Tetta C et al (2014) Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev 23(15):1809–1819
Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C et al (2011) Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 26(5):1474–1483
Wang R, Lin M, Li L, Li L, Qi G, Rong R et al (2014) Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi 94(42):3298–3303
Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G et al (2016) Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res 8(10):4289–4299
Ju GQ, Cheng J, Zhong L, Wu S, Zou XY, Zhang GY et al (2015) Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE 10(3):e0121534
Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G et al (2014) Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther 5(2):40
Aghajani Nargesi A, Lerman LO, Eirin A (2017) Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther 8(1):273
Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R et al (2017) Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A 23(21–22):1262–1273
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y et al (2013) Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4(2):34
Ezquer F, Ezquer M, Simon V, Pardo F, Yañez A, Carpio D et al (2009) Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Trans 15(11):1354–1365
Fang Y, Tian X, Bai S, Fan J, Hou W, Tong H et al (2012) Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med 30(1):85–92
Abdel Aziz MT, Wassef MA, Ahmed HH, Rashed L, Mahfouz S, Aly MI et al (2014) The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metab Syndr 6(1):34
Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P et al (2010) Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol 114(3):e107–e116
Tian H, Lu Y, Shah SP, Wang Q, Hong S (2012) 14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury. Stem Cells Dev 21(7):1187–1199
Masoud MS, Anwar SS, Afzal MZ, Mehmood A, Khan SN, Riazuddin S (2012) Pre-conditioned mesenchymal stem cells ameliorate renal ischemic injury in rats by augmented survival and engraftment. J Transl Med 10:243
Altun B, Yilmaz R, Aki T, Akoglu H, Zeybek D, Piskinpasa S et al (2012) Use of mesenchymal stem cells and darbepoetin improve ischemia-induced acute kidney injury outcomes. Am J Nephrol 35(6):531–539
Cai J, Yu X, Zhang B, Zhang H, Fang Y, Liu S et al (2014) Atorvastatin improves survival of implanted stem cells in a rat model of renal ischemia-reperfusion injury. Am J Nephrol 39(6):466–475
Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F et al (2008) Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells 26(7):1749–1757
George SK, Abolbashari M, Jackson JD, Aboushwareb T, Atala A, Yoo JJ (2016) Potential use of autologous renal cells from diseased kidneys for the treatment of renal failure. PLoS ONE 11(10):e0164997
Stenvinkel P, Wadstrom J, Bertram T, Detwiler R, Gerber D, Brismar TB et al (2016) Implantation of autologous selected renal cells in diabetic chronic kidney disease stages 3 and 4 clinical experience of a “first in human” study. Kidney Int Rep 1(3):105–113
Stavas J, Filler G, Jain D, Ludlow J, Basu J, Payne R et al (2022) Renal autologous cell therapy to stabilize function in diabetes-related chronic kidney disease: corroboration of mechanistic action with cell marker analysis. Kidney Int Rep 7(7):1619–1629
Hickson LJ, Eirin A, Lerman LO (2016) Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int 89(4):767–778
Acknowledgements
All figures are crafted at biorender.com
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Contributed substantially to the conception or design of the work: Mehmet Kanbay, Sidar Copur. Drafted the manuscript: Sidar Copur, Furkan Yvuz. Revision of the manuscript for important intellectual content: Mehmet Kanbay, Adrian Covic
Corresponding author
Ethics declarations
Conflict of interest
All the authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Copur, S., Yavuz, F., Covic, A. et al. A review on renal autologous cell transplantation: an investigational approach towards chronic kidney disease. Int Urol Nephrol 55, 2539–2544 (2023). https://doi.org/10.1007/s11255-023-03574-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03574-5