Abstract
Objective
To mechanistically assess the involvement of tenascin-C (TNC) in diabetic nephropathy (DN).
Methods
Renal specimens from DN patients were histopathologically examined, and their TNC expression patterns were evaluated via immunohistochemistry. Additionally, the hereditarily diabetic C57BL/KsJ db/db mice were induced to develop DN via adaptive feeding, and then their renal levels of TNC and β-catenin were assessed via western blotting and immunohistochemistry. Furthermore, the TNC and β-catenin levels in primary rat mesangial cells (RMCs) cultured with high glucose levels were assessed via western blotting. In parallel, RMCs cultured with TNC in the presence or absence of the β-catenin blocker ICG-001 were analyzed for their fibronectin and collagen I levels via immunostaining, and for their fibronectin, α-SMA, vimentin, PDGFR-β, PCNA, and β-catenin levels via western blotting.
Results
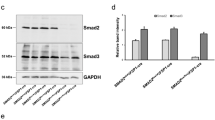
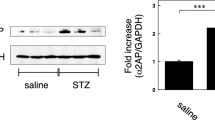
The TNC levels in the specimens were associated with the pathological classification. In these DN specimens, TNC protein was highly detected in the MCs and slightly in the tubulointerstitium. Renal TNC (P < 0.05) and β-catenin (P < 0.001) were upregulated in db/db vs. db/m mice. High-glucose treatment upregulated TNC (P < 0.01) and β-catenin (P < 0.05) in RMCs. TNC treatment upregulated fibronectin (P < 0.05), α-SMA (P < 0.01), vimentin (P < 0.05), PCNA (P < 0.05), and β-catenin (P < 0.05) in RMCs, as assessed via western blotting. Immunohistochemical analysis confirmed the fibronectin upregulation and showed collagen I upregulation. Western-blot results also showed that levels of fibronectin (P < 0.001), α-SMA (P < 0.01), vimentin (P < 0.001), PCNA (P < 0.05), PDGFR-β (P < 0.05), and β-catenin (P < 0.01) were lower in RMCs co-treated with TNC and ICG-001 than in TNC-treated cells. Immunofluorescence analysis confirmed the decreased fibronectin level and showed that the collagen I level was also decreased by ICG-001.
Conclusion
TNC is upregulated in DN and induces MC proliferation and fibrosis through the β-catenin pathway.





Similar content being viewed by others
Data availability
Inquiries for any data unavailable in the text can be directed to the corresponding author.
References
Anders H, Huber T, Isermann B, Schiffer M (2018) CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 14:361–377. https://doi.org/10.1038/s41581-018-0001-y
Tung C, Hsu Y, Shih Y, Chang P, Lin C (2018) Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology (Carlton). https://doi.org/10.1111/nep.13451
Tucker R, Chiquet-Ehrismann R (2015) Tenascin-C: its functions as an integrin ligand. Int J Biochem Cell Biol 65:165–168. https://doi.org/10.1016/j.biocel.2015.06.003
Fu H, Tian Y, Zhou L, Zhou D, Tan R, Stolz D, Liu Y (2017) Tenascin-C Is a Major Component of the Fibrogenic Niche in Kidney Fibrosis. J Am Soc Nephrol 28:785–801. https://doi.org/10.1681/asn.2016020165
Liabeuf S, Barreto D, Kretschmer A, Barreto F, Renard C, Andrejak M, Massy Z (2011) High circulating levels of large splice variants of tenascin-C is associated with mortality and cardiovascular disease in chronic kidney disease patients. Atherosclerosis 215:116–124. https://doi.org/10.1016/j.atherosclerosis.2010.11.038
Imanaka-Yoshida K, Tawara I, Yoshida T (2020) Tenascin-C in cardiac disease: a sophisticated controller of inflammation, repair, and fibrosis. Am J Physiol Cell Physiol 319:C781–C796. https://doi.org/10.1152/ajpcell.00353.2020
Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, Varga J (2016) Tenascin-C drives persistence of organ fibrosis. Nat Commun 7:11703. https://doi.org/10.1038/ncomms11703
Pang X, Zhang Y, Shi X, Li D, Han J (2018) ERp44 depletion exacerbates ER stress and aggravates diabetic nephropathy in db/db mice. Biochem Biophys Res Commun 504:921–926. https://doi.org/10.1016/j.bbrc.2018.09.037
Zhou X, Liu Z, Ying K, Wang H, Liu P, Ji X, He Z (2020) WJ-39, an Aldose Reductase Inhibitor, Ameliorates Renal Lesions in Diabetic Nephropathy by Activating Nrf2 Signaling. Oxid Med Cell Longev 2020:7950457. https://doi.org/10.1155/2020/7950457
Chen S, Fu H, Wu S, Zhu W, Liao J, Hong X, Liu Y (2019) Tenascin-C protects against acute kidney injury by recruiting Wnt ligands. Kidney Int 95:62–74. https://doi.org/10.1016/j.kint.2018.08.029
Fang X, Hu J, Zhou H (2020) Knock-Down of Long Non-Coding RNA ANRIL Suppresses Mouse Mesangial Cell Proliferation, Fibrosis, Inflammation via Regulating Wnt/β-Catenin and MEK/ERK Pathways in Diabetic Nephropathy. Exp Clin Endocrinol Diabetes.https://doi.org/10.1055/a-1185-9283.
Saupe F, Schwenzer A, Jia Y, Gasser I, Spenlé C, Langlois B, Orend G (2013) Tenascin-C downregulates wnt inhibitor dickkopf-1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep 5:482–492. https://doi.org/10.1016/j.celrep.2013.09.014
Hendaoui I, Tucker R, Zingg D, Bichet S, Schittny J, Chiquet-Ehrismann R (2014) Tenascin-C is required for normal Wnt/β-catenin signaling in the whisker follicle stem cell niche. Matrix Biol 40:46–53. https://doi.org/10.1016/j.matbio.2014.08.017
Xu W, Guan M, Zheng Z, Gao F, Zeng Y, Qin Y, Xue Y (2014) Exendin-4 alleviates high glucose-induced rat mesangial cell dysfunction through the AMPK pathway. Cell Physiol Biochem 33:423–432. https://doi.org/10.1159/000358623
Cai M, Bompada P, Atac D, Laakso M, Groop L, De Marinis Y (2016) Epigenetic regulation of glucose-stimulated osteopontin (OPN) expression in diabetic kidney. Biochem Biophys Res Commun 469:108–113. https://doi.org/10.1016/j.bbrc.2015.11.079
Tervaert T, Mooyaart A, Amann K, Cohen A, Cook H, Drachenberg C, Bruijn J (2010) Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21:556–563. https://doi.org/10.1681/asn.2010010010
Wang J, Yang Q, Nie Y, Guo H, Zhang F, Zhou X, Yin X (2017) Tetrahydrobiopterin contributes to the proliferation of mesangial cells and accumulation of extracellular matrix in early-stage diabetic nephropathy. J Pharm Pharmacol 69:182–190. https://doi.org/10.1111/jphp.12677
Zou C, Xie R, Bao Y, Liu X, Sui M, Suhong M, Li S, Yin H (2013) Iron chelator alleviates tubulointerstitial fibrosis in diabetic nephropathy rats by inhibiting the expression of tenascinC and other correlation factors. Endocrine 44:666–674. https://doi.org/10.1007/s12020-013-9907-0
He W, Dai C, Li Y, Zeng G, Monga S, Liu Y (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20:765–776. https://doi.org/10.1681/asn.2008060566
Shi M, Tian P, Liu Z, Zhang F, Zhang Y, Qu L, Guo B (2020) MicroRNA-27a targets Sfrp1 to induce renal fibrosis in diabetic nephropathy by activating Wnt/β-Catenin signalling. Biosci Rep. https://doi.org/10.1042/bsr20192794
Shimojo N, Hashizume R, Kanayama K, Hara M, Suzuki Y, Nishioka T, Imanaka-Yoshida K (2015) Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin αVβ3/nuclear factor-κB/interleukin-6 axis. Hypertension 66:757–766. https://doi.org/10.1161/hypertensionaha.115.06004
Ishigaki T, Imanaka-Yoshida K, Shimojo N, Matsushima S, Taki W, Yoshida T (2011) Tenascin-C enhances crosstalk signaling of integrin αvβ3/PDGFR-β complex by SRC recruitment promoting PDGF-induced proliferation and migration in smooth muscle cells. J Cell Physiol 226:2617–2624. https://doi.org/10.1002/jcp.22614
Buelli S, Rosanò L, Gagliardini E, Corna D, Longaretti L, Pezzotta A, Benigni A (2014) β-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol 25:523–533. https://doi.org/10.1681/asn.2013040362
Ridgway R, Serrels B, Mason S, Kinnaird A, Muir M, Patel H, Brunton V (2012) Focal adhesion kinase is required for β-catenin-induced mobilization of epidermal stem cells. Carcinogenesis 33:2369–2376. https://doi.org/10.1093/carcin/bgs284
Despeaux M, Chicanne G, Rouer E, De Toni-Costes F, Bertrand J, Mansat-De Mas V, Racaud-Sultan C (2012) Focal adhesion kinase splice variants maintain primitive acute myeloid leukemia cells through altered Wnt signaling. Stem Cells 30:1597–1610. https://doi.org/10.1002/stem.1157
Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr H, Delaloye J, Huelsken J (2011) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481:85–89. https://doi.org/10.1038/nature10694
Lan X, Wen H, Aslam R, Shoshtari S, Mishra A, Kumar V, Singhal P (2018) Nicotine enhances mesangial cell proliferation and fibronectin production in high glucose milieu via activation of Wnt/β-catenin pathway. Biosci Rep. https://doi.org/10.1042/bsr20180100
Acknowledgements
Our thanks go to all laboratory members who helped to carry out this project.
Funding
This work was supported by the National Natural Science Foundation of China Grant 81900627, Guangxi Natural Science Foundation grant 2021GXNSFAA196057, Guangxi Administration of Traditional Chinese Medicine grant GZZC2019107 and Guangxi Science and Technology Base and Talent Project grant 2022AC04001.
Author information
Authors and Affiliations
Contributions
H-XT, H-CH, L-SL, G-HF, X-SW, Y-HF, Z-J, C-SQ, and G-HJ designed and performed the experiments, and collected and analyzed the data. C-SQ helped in performing the experiments and collecting the data. P-XX and C-SQ wrote the manuscript draft. C-SQ analyzed the data and prepared the figures. G-HJ finished the final touch-up of the article. All the authors contributed to the article and approved the submitted version.111.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, X., Hou, X., Hu, C. et al. Tenascin-C promotes the proliferation and fibrosis of mesangial cells in diabetic nephropathy through the β-catenin pathway. Int Urol Nephrol 55, 2507–2516 (2023). https://doi.org/10.1007/s11255-023-03547-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03547-8