Abstract
Background
Patients with end-stage renal failure (ESRD) or dialysis frequently suffer from secondary hyperparathyroidism (sHPTH), a severe complication of mineral metabolism disorders. The calcimimetic etelcalcetide has been approved and shown efficacy in randomized controlled trials, however, data are limited from real-life studies.
This study aimed to evaluate the long-term use etelcalcetide for the treatment of sHPTH (PTH > 600 pg/mL) in patients undergoing extracorporeal hemodialysis for ESRD for at least 2 years.
Methods
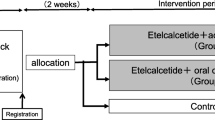
In 45 patients, we administered etelcalcetide for the treatment of sHPTH (PTH > 600 pg/mL); One group of patients (control group, Group A; N = 26) were previously treated with intravenous vitamin D analogues only (paricalcitol 5 µg/ml, three times/week) and then treated with etelcalcetide and a second group of patients already on cinacalcet therapy for at least six months in combination with iv paricalcitol were switched to etelcalcetide (Group B, N = 19).
Results
PTH levels decreased over time in both groups of patients, with higher values for patients previously treated with cinacalcet (Group B) compared to Group A for the entire study duration even if the final value of the two groups was comparable. After 12 months, the percentage of subjects who had PTH concentrations within the targets recommended by KDIGO guidelines was 87% in Group A and 58% in Group B. In seven patients, despite a parathyroid gland volume > 1000 mm3, an adequate response in the reduction of PTH was obtained.
Conclusion
Findings from this study demonstrate that the efficacy of etelcalcetide is maintained over the long term.



Similar content being viewed by others
Data availability
The data supporting our fndings are presented in the article. The datasets of the current study are available from the corresponding author on reasonable request.
References
Kestenbaum B, Belozeroff V (2007) Mineral metabolism disturbances in patients with chronic kidney disease. Eur J Clin Invest 37:607–622. https://doi.org/10.1111/j.1365-2362.2007.01840.x
Goodman WG (2004) The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial 17:209–216. https://doi.org/10.1111/j.0894-0959.2004.17308.x
Rodriguez M, Canalejo A, Garfia B et al (2002) Pathogenesis of refractory secondary hyperparathyroidism. Kidney Int Suppl. https://doi.org/10.1046/j.1523-1755.61.s80.26.x
Cozzolino M, Olivi L, Voli E et al (2009) PREVENZIONE E TRATTAMENTO DELL’IPERPARATIROIDISMO SECONDARIO NEL PAZIENTE CON MALATTIA RENALE CRONICA ALLO STADIO NON IN DIALISI. G Ital Nefrol 26(S49):S30–S35
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. https://doi.org/10.1038/ki.2009.188
Disease K (2011) Improving global outcomes (KDIGO) CKD-MBD update work group (2017) KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 7:1–59. https://doi.org/10.1016/j.kisu.2017.04.001
Ketteler M, Block GA, Evenepoel P et al (2017) Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int 92:26–36. https://doi.org/10.1016/j.kint.2017.04.006
Isakova T, Nickolas TL, Denburg M et al (2017) KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis 70:737–751. https://doi.org/10.1053/j.ajkd.2017.07.019
Drüeke TB (2000) Hyperparathyroidism in Chronic Kidney Disease. In: Anawalt B, Boyce A et al (eds) Feingold KR. Endotext. MDText.com Inc, South Dartmouth (MA)
Rodríguez-Ortiz ME, Rodríguez M (2020) Recent advances in understanding and managing secondary hyperparathyroidism in chronic kidney disease. FRes. https://doi.org/10.12688/f1000research.22636.1
Friedl C, Zitt E (2018) Role of etelcalcetide in the management of secondary hyperparathyroidism in hemodialysis patients: a review on current data and place in therapy. Drug Des Devel Ther 12:1589–1598. https://doi.org/10.2147/DDDT.S134103
Fliser D, Kollerits B, Neyer U et al (2007) Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the mild to moderate kidney disease (MMKD) Study. J Am Soc Nephrol 18:2600–2608. https://doi.org/10.1681/ASN.2006080936
Tentori F, Wang M, Bieber BA et al (2015) Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 10:98–109. https://doi.org/10.2215/CJN.12941213
Walter S, Baruch A, Dong J et al (2013) Pharmacology of AMG 416 (Velcalcetide), a novel peptide agonist of the calcium-sensing receptor, for the treatment of secondary hyperparathyroidism in hemodialysis patients. J Pharmacol Exp Ther 346:229–240. https://doi.org/10.1124/jpet.113.204834
Walter S, Baruch A, Alexander ST et al (2014) Comparison of AMG 416 and cinacalcet in rodent models of uremia. BMC Nephrol 15:81. https://doi.org/10.1186/1471-2369-15-81
PubChem Etelcalcetide. https://pubchem.ncbi.nlm.nih.gov/compound/71511839. Accessed 12 June 2021
Alexander ST, Hunter T, Walter S et al (2015) Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol Pharmacol 88:853–865. https://doi.org/10.1124/mol.115.098392
Chen P, Melhem M, Xiao J et al (2015) Population pharmacokinetics analysis of AMG 416, an allosteric activator of the calcium-sensing receptor, in subjects with secondary hyperparathyroidism receiving hemodialysis. J Clin Pharmacol 55:620–628. https://doi.org/10.1002/jcph.460
Pereira L, Meng C, Marques D, Frazão JM (2018) Old and new calcimimetics for treatment of secondary hyperparathyroidism: impact on biochemical and relevant clinical outcomes. Clin Kidney J 11:80–88. https://doi.org/10.1093/ckj/sfx125
Block GA, Bushinsky DA, Cunningham J et al (2017) Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA 317:146–155. https://doi.org/10.1001/jama.2016.19456
Bushinsky DA, Chertow GM, Cheng S et al (2020) One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism. Nephrol Dial Transplant 35:1769–1778. https://doi.org/10.1093/ndt/gfz039
Itano Y, Kato S, Tsuboi M et al (2020) A prospective, randomized clinical trial of etelcalcetide in patients receiving hemodialysis with secondary hyperparathyroidism (the DUET Trial). Kidney Int Rep 5:2168–2177. https://doi.org/10.1016/j.ekir.2020.09.010
Fukagawa M, Yokoyama K, Shigematsu T et al (2017) A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients. Nephrol Dial Transplant 32:1723–1730. https://doi.org/10.1093/ndt/gfw408
Russo D, Tripepi R, Malberti F et al (2019) Etelcalcetide in patients on hemodialysis with severe secondary hyperparathyroidism multicenter study in “real life.” J Clin Med. https://doi.org/10.3390/jcm8071066
Anonymous (2018) Parsabiv. In: European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/parsabiv. Accessed 12 June 2021
Shigematsu T, Fukagawa M, Yokoyama K et al (2018) Long-term effects of etelcalcetide as intravenous calcimimetic therapy in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 22:426–436. https://doi.org/10.1007/s10157-017-1442-5
Piccoli GB, Trabace T, Chatrenet A et al (2020) New intravenous calcimimetic agents: new options, new problems. An example on how clinical economical and ethical considerations affect choice of treatment. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17041238
Makady A, de Boer A, Hillege H et al (2017) What Is Real-World Data? A review of definitions based on literature and stakeholder interviews. Value Health 20:858–865. https://doi.org/10.1016/j.jval.2017.03.008
Eichler H-G, Pignatti F, Schwarzer-Daum B et al (2021) Randomized controlled trials versus real world evidence: neither magic nor myth. Clin Pharmacol Ther 109:1212–1218. https://doi.org/10.1002/cpt.2083
Garrison LP, Neumann PJ, Erickson P et al (2007) Using real-world data for coverage and payment decisions: the ISPOR real-world data task force report. Value Health 10:326–335. https://doi.org/10.1111/j.1524-4733.2007.00186.x
Block GA, Klassen PS, Lazarus JM et al (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15:2208–2218. https://doi.org/10.1097/01.ASN.0000133041.27682.A2
Chen J, Budoff MJ, Reilly MP et al (2017) Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2:635–643. https://doi.org/10.1001/jamacardio.2017.0363
Petrović D, Stojimirović B (2008) Cardiovascular morbidity and mortality in patients treated with hemodialysis–epidemiological analysis. Vojnosanit Pregl 65:893–900. https://doi.org/10.2298/vsp0812893p
Acknowledgements
Authors wish to thank Dr Colin Gerard Egan (CE Medical Writing SRLS, Pisa, Italy) for revising the manuscript.
Author information
Authors and Affiliations
Contributions
All authors MM, LJ, LZ, EA, IP, contributed equally to each of the following (a-f). (a) the design of the study, (b) collected data, (c) performed statistical analysis (d) interpreted results (e) wrote the manuscript and (f) read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morosetti, M., Jankovic, L., Zappalà, L. et al. Long-term use of etelcalcetide for the treatment of secondary hyperparathyroidism in patients undergoing hemodialysis for end-stage renal failure: a real-life retrospective observational study. Int Urol Nephrol 55, 1865–1873 (2023). https://doi.org/10.1007/s11255-023-03505-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03505-4