Abstract
Purpose
To assess adherence to combination therapy comprising α-adrenergic blocker (AB) and 5α-reductase inhibitor (5ARI) for benign prostatic hyperplasia (BPH) in a real-world setting and whether lower urinary tract symptoms (LUTS) will relapse after discontinuing one medication from long-term combination therapy.
Methods
BPH/LUTS patients receiving initial AB +5ARI combination therapy for at least 1 year between January 2012 and January 2017 were retrospectively analyzed. The patients were classified into DC-AB group (n = 65, AB discontinued) and DC-5ARI group (n = 77, 5ARI discontinued) and followed up. Clinical effects were assessed at baseline and annually using the International Prostatic Symptoms Score (IPSS), quality of life (QoL) index, total prostate volume (TPV), maximal flow rate (Qmax), and prostate-specific antigen (PSA) level.
Results
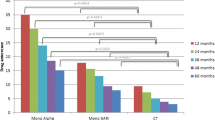
Of total 1783 patients, 809 (45.4%) patients were identified with more than 1-year combination therapy. After withdrawal of one medication from combination therapy, the TPV progression (27.6% vs. − 10.8%; P < 0.001) and the requirement for prostate surgery (14.3% vs. 6.1%; P = 0.038) were significantly higher in the DC-5ARI group than in the DC-AB group. The rate of resuming combination therapy was significantly higher in the DC-5ARI group than in the DC-AB group (38.9% vs. 23.0%; P = 0.009).
Conclusions
Adherence to combination BPH therapy is relatively low. Although patients adhered to combination therapy for more than 1 year, a higher risk of requiring prostate surgery or resuming combination therapy was observed in patients who discontinued 5ARI.

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Emberton M, Andriole GL, de la Rosette J, Djavan B, Hoefner K, Vela Navarrete R, Nordling J, Roehrborn C, Schulman C, Teillac P, Tubaro A, Nickel JC (2003) Benign prostatic hyperplasia: a progressive disease of aging men. Urology 61(2):267–273. https://doi.org/10.1016/s0090-4295(02)02371-3
Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, Lieber MM (1997) Natural history of prostatism: risk factors for acute urinary retention. J Urol 158(2):481–487. https://doi.org/10.1016/s0022-5347(01)64508-7
Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG (2006) The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol 176(3):868–874. https://doi.org/10.1016/j.juro.2006.04.024
Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G (2007) The long-term outcome of medical therapy for BPH. Eur Urol 51(6):1522–1533. https://doi.org/10.1016/j.eururo.2007.03.034
Fourcade RO, Lacoin F, Rouprêt M, Slama A, Le Fur C, Michel E, Sitbon A, Cotté FE (2012) Outcomes and general health-related quality of life among patients medically treated in general daily practice for lower urinary tract symptoms due to benign prostatic hyperplasia. World J Urol 30(3):419–426. https://doi.org/10.1007/s00345-011-0756-2
Strope SA, Elliott SP, Saigal CS, Smith A, Wilt TJ, Wei JT (2011) Urologist compliance with AUA best practice guidelines for benign prostatic hyperplasia in medicare population. Urology 78(1):3–9. https://doi.org/10.1016/j.urology.2010.12.087
De Nunzio C, Tubaro A (2011) BPH: unmet needs in managing LUTS–a European perspective. Nat Rev Urol 9(1):9–10. https://doi.org/10.1038/nrurol.2011.190
Becher E, Roehrborn CG, Siami P, Gagnier RP, Wilson TH, Montorsi F (2009) The effects of dutasteride, tamsulosin, and the combination on storage and voiding in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the Combination of Avodart and Tamsulosin study. Prostate Cancer Prostatic Dis 12(4):369–374. https://doi.org/10.1038/pcan.2009.37
Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, Morrill BB, Gagnier RP, Montorsi F (2010) The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 57(1):123–131. https://doi.org/10.1016/j.eururo.2009.09.035
Terris MK, Stamey TA (1991) Determination of prostate volume by transrectal ultrasound. J Urol 145(5):984–987. https://doi.org/10.1016/s0022-5347(17)38508-7
McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM Jr, Clarke HS, Crawford ED, Diokno A, Foley JP, Foster HE, Jacobs SC, Kaplan SA, Kreder KJ, Lieber MM, Lucia MS, Miller GJ, Menon M, Milam DF, Ramsdell JW, Schenkman NS, Slawin KM, Smith JA (2003) The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 349(25):2387–2398. https://doi.org/10.1056/NEJMoă56
Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Morrill B, Montorsi F (2008) The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 179(2):616–621. https://doi.org/10.1016/j.juro.2007.09.084. (discussion 621)
Lee JW, Kim JH (2022) Drug prescription patterns during initial treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia: a study based on health insurance review and assessment database. J Korean Med Sci 37(12):e95. https://doi.org/10.3346/jkms.2022.37.e95
Gruschkus S, Poston S, Eaddy M, Chaudhari S (2012) Adherence to 5-alpha reductase inhibitor therapy for benign prostatic hyperplasia: clinical and economic outcomes. P t 37(8):464–470
Krueger KP, Berger BA, Felkey B (2005) Medication adherence and persistence: a comprehensive review. Adv Ther 22(4):313–356. https://doi.org/10.1007/bf02850081
Eisen C, Lulic Z, Palacios-Moreno JM, Adalig B, Hennig M, Cortes V, Gilg F, Kostev K (2020) Persistence and adherence to dutasteride/tamsulosin fixed-dose versus free-combination alpha blocker/5ARI therapy in patients with benign prostate hyperplasia in Germany. Int J Clin Pharmacol Ther 58(1):37–49. https://doi.org/10.5414/cp203549
Cindolo L, Pirozzi L, Fanizza C, Romero M, Tubaro A, Autorino R, De Nunzio C, Schips L (2015) Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol 68(3):418–425. https://doi.org/10.1016/j.eururo.2014.11.006
Matsukawa Y, Takai S, Funahashi Y, Majima T, Kato M, Yamamoto T, Gotoh M (2017) Effects of withdrawing α1-blocker from combination therapy with α1-blocker and 5α-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol 198(4):905–912. https://doi.org/10.1016/j.juro.2017.05.031
Kim HW, Moon DG, Kim HM, Hwang JH, Kim SC, Nam SG, Park JT (2011) Effect of shifting from combination therapy to monotherapy of α-blockers or 5α-reductase inhibitors on prostate volume and symptoms in patients with benign prostatic hyperplasia. Korean J Urol 52(10):681–686. https://doi.org/10.4111/kju.2011.52.10.681
Lin VC, Liao CH, Kuo HC (2014) Progression of lower urinary tract symptoms after discontinuation of 1 medication from 2-year combined alpha-blocker and 5-alpha-reductase inhibitor therapy for benign prostatic hyperplasia in men–a randomized multicenter study. Urology 83(2):416–421. https://doi.org/10.1016/j.urology.2013.09.036
Kim W, Jung JH, Kang TW, Song JM, Chung HC (2014) Clinical effects of discontinuing 5-alpha reductase inhibitor in patients with benign prostatic hyperplasia. Korean J Urol 55(1):52–56. https://doi.org/10.4111/kju.2014.55.1.52
Emberton M, Fitzpatrick JM, Rees J (2011) Risk stratification for benign prostatic hyperplasia (BPH) treatment. BJU Int 107(6):876–880. https://doi.org/10.1111/j.1464-410X.2010.10041.x
Acknowledgements
We thank Hye Young Han from Nowon Eulji University Hospital Library for manuscript’s technical editing.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
JDC: conceptualized the study. JDC: designed the study. TKY, JYK and HDJ: collected the original data. JHL: performed the statistical analysis of the data. JDC and JHL: performed the writing of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was approved by Nowon Eulji University Hospital, Eulji University School of Medicine Ethical Committee, seoul, South Korea (EMC-2021-05-021). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, J.D., Yoo, T.K., Kang, J.Y. et al. Clinical outcomes of withdrawing one medication from long-term combination therapy comprising α-blocker and 5α-reductase inhibitor for benign prostatic hyperplasia. Int Urol Nephrol 55, 845–851 (2023). https://doi.org/10.1007/s11255-023-03476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03476-6