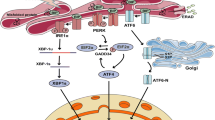
Abstract
Background
Diabetic nephropathy (DN) is a kidney disease resulting from diabetes. Macrophages and macrophage-mediated inflammation contributed to the development of DN. Tripartite motif containing 23 (TRIM23) is E3 ligase and has been involved in inflammation. Until now, the precise roles of TRIM23 in DN are not described yet. Therefore, we evaluated the functions of TRIM23 in DN.
Methods
We generated mice with TRIM23 deficiency in macrophages. We also established diabetic mice model. The expression of TRIM23 was measured in diabetic animals. The DN symptoms were compared between diabetic wild-type (WT) mice and TRIM23 conditional knock out mice. The cytokine expression, ubiquitination of TAB2, and interactions between TAB2 and IKK were compared in oxidized low-density lipoprotein (oxLDL) or lipopolysaccharides (LPS)-treated WT and TRIM23-deficient macrophages.
Results
Upregulation of TRIM23 was observed in diabetic mice and LPS or oxLDL-treated macrophages. Diabetic mice with TRIM23 deficiency in macrophages had attenuated DN symptoms. TRIM23-deficient macrophages had decreased pro-inflammatory cytokines production after oxLDL or LPS stimulation. TRIM23 was predicted to interact with TAB2. The ubiquitination of TAB2 was abolished in oxLDL-treated TRIM23-deficient macrophages, which correlated with decreased activation of IKK.
Conclusion
TRIM23 regulates inflammation in macrophages and plays important role in DN.




Similar content being viewed by others
Data availability
Data could be obtained upon reasonable request to the corresponding author.
References
Lim A (2014) Diabetic nephropathy: Complications and treatment. Int J Nephrol Renovasc Dis 7:361–381. https://doi.org/10.2147/IJNRD.S40172
Varghese RT, Jialal I (2022) Diabetic nephropathy. In: StatPearls. Treasure Island (FL)
Chang DY, Li MR, Yu XJ, Wang SX, Chen M, Zhao MH (2021) Clinical and pathological characteristics of patients with nonproteinuric diabetic nephropathy. Front Endocrinol (Lausanne) 12:761386. https://doi.org/10.3389/fendo.2021.761386
Fornoni A, Ijaz A, Tejada T, Lenz O (2008) Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4(1):10–17. https://doi.org/10.2174/157339908783502361
Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm 2012:146154. https://doi.org/10.1155/2012/146154
Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, Yoshinaga K (1993) The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis 21(5):480–485. https://doi.org/10.1016/s0272-6386(12)80393-3
Sassy-Prigent C, Heudes D, Mandet C, Belair MF, Michel O, Perdereau B, Bariety J, Bruneval P (2000) Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 49(3):466–475. https://doi.org/10.2337/diabetes.49.3.466
Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH (2004) Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int 65(1):116–128. https://doi.org/10.1111/j.1523-1755.2004.00367.x
Navarro-Gonzalez JF, Mora-Fernandez C (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19(3):433–442. https://doi.org/10.1681/ASN.2007091048
Yang K, Wang XQ, He YS, Lu L, Chen QJ, Liu J, Shen WF (2010) Advanced glycation end products induce chemokine/cytokine production via activation of p38 pathway and inhibit proliferation and migration of bone marrow mesenchymal stem cells. Cardiovasc Diabetol 9:66. https://doi.org/10.1186/1475-2840-9-66
Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ (2003) Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int 63(1):83–95. https://doi.org/10.1046/j.1523-1755.2003.00717.x
Tesch GH (2010) Macrophages and diabetic nephropathy. Semin Nephrol 30(3):290–301. https://doi.org/10.1016/j.semnephrol.2010.03.007
Meroni G, Diez-Roux G (2005) TRIM/RBCC, a novel class of “single protein RING finger” E3 ubiquitin ligases. BioEssays 27(11):1147–1157. https://doi.org/10.1002/bies.20304
Arimoto K, Funami K, Saeki Y, Tanaka K, Okawa K, Takeuchi O, Akira S, Murakami Y, Shimotohno K (2010) Polyubiquitin conjugation to NEMO by triparite motif protein 23 (TRIM23) is critical in antiviral defense. Proc Natl Acad Sci USA 107(36):15856–15861. https://doi.org/10.1073/pnas.1004621107
Sparrer KMJ, Gableske S, Zurenski MA, Parker ZM, Full F, Baumgart GJ, Kato J, Pacheco-Rodriguez G, Liang C, Pornillos O, Moss J, Vaughan M, Gack MU (2017) TRIM23 mediates virus-induced autophagy via activation of TBK1. Nat Microbiol 2(11):1543–1557. https://doi.org/10.1038/s41564-017-0017-2
Poole E, Groves I, MacDonald A, Pang Y, Alcami A, Sinclair J (2009) Identification of TRIM23 as a cofactor involved in the regulation of NF-kappaB by human cytomegalovirus. J Virol 83(8):3581–3590. https://doi.org/10.1128/JVI.02072-08
Lei L, Bandola-Simon J, Roche PA (2018) Ubiquitin-conjugating enzyme E2 D1 (Ube2D1) mediates lysine-independent ubiquitination of the E3 ubiquitin ligase March-I. J Biol Chem 293(11):3904–3912. https://doi.org/10.1074/jbc.RA117.001322
Cho KJ, Walseng E, Ishido S, Roche PA (2015) Ubiquitination by March-I prevents MHC class II recycling and promotes MHC class II turnover in antigen-presenting cells. Proc Natl Acad Sci USA 112(33):10449–10454. https://doi.org/10.1073/pnas.1507981112
Sha H, Zeng H, Zhao J, Jin H (2019) Mangiferin ameliorates gestational diabetes mellitus-induced placental oxidative stress, inflammation and endoplasmic reticulum stress and improves fetal outcomes in mice. Eur J Pharmacol 859:172522. https://doi.org/10.1016/j.ejphar.2019.172522
Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ (2004) TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell 15(4):535–548. https://doi.org/10.1016/j.molcel.2004.08.008
Dawidziak DM, Sanchez JG, Wagner JM, Ganser-Pornillos BK, Pornillos O (2017) Structure and catalytic activation of the TRIM23 RING E3 ubiquitin ligase. Proteins 85(10):1957–1961. https://doi.org/10.1002/prot.25348
Xu YR, Lei CQ (2020) TAK1-TABs complex: A central signalosome in inflammatory responses. Front Immunol 11:608976. https://doi.org/10.3389/fimmu.2020.608976
Chin MP, Bakris GL, Block GA, Chertow GM, Goldsberry A, Inker LA, Heerspink HJL, O’Grady M, Pergola PE, Wanner C, Warnock DG, Meyer CJ (2018) Bardoxolone methyl improves kidney function in patients with chronic kidney disease stage 4 and type 2 diabetes: Post-hoc analyses from bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes study. Am J Nephrol 47(1):40–47. https://doi.org/10.1159/000486398
Perez-Gomez MV, Sanchez-Nino MD, Sanz AB, Zheng B, Martin-Cleary C, Ruiz-Ortega M, Ortiz A, Fernandez-Fernandez B (2016) Targeting inflammation in diabetic kidney disease: Early clinical trials. Expert Opin Investig Drugs 25(9):1045–1058. https://doi.org/10.1080/13543784.2016.1196184
Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M (1991) Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int 40(6):1007–1012. https://doi.org/10.1038/ki.1991.308
Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ (2006) Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 11(3):226–231. https://doi.org/10.1111/j.1440-1797.2006.00576.x
Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A (2006) Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci 1084:89–117. https://doi.org/10.1196/annals.1372.039
Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K, Yano Y (1998) Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83(3):859–862. https://doi.org/10.1210/jcem.83.3.4618
Chen YL, Qiao YC, Xu Y, Ling W, Pan YH, Huang YC, Geng LJ, Zhao HL, Zhang XX (2017) Serum TNF-alpha concentrations in type 2 diabetes mellitus patients and diabetic nephropathy patients: A systematic review and meta-analysis. Immunol Lett 186:52–58. https://doi.org/10.1016/j.imlet.2017.04.003
Moriwaki Y, Inokuchi T, Yamamoto A, Ka T, Tsutsumi Z, Takahashi S, Yamamoto T (2007) Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol 44(4):215–218. https://doi.org/10.1007/s00592-007-0007-6
Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS (2012) Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23(3):507–515. https://doi.org/10.1681/ASN.2011060627
Lei Y, Devarapu SK, Motrapu M, Cohen CD, Lindenmeyer MT, Moll S, Kumar SV, Anders HJ (2019) Interleukin-1beta inhibition for chronic kidney disease in obese mice with type 2 diabetes. Front Immunol 10:1223. https://doi.org/10.3389/fimmu.2019.01223
Feigerlova E, Battaglia-Hsu SF (2017) IL-6 signaling in diabetic nephropathy: From pathophysiology to therapeutic perspectives. Cytokine Growth Factor Rev 37:57–65. https://doi.org/10.1016/j.cytogfr.2017.03.003
Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A (2010) NF-kappaB in renal inflammation. J Am Soc Nephrol 21(8):1254–1262. https://doi.org/10.1681/ASN.2010020218
Starkey JM, Haidacher SJ, LeJeune WS, Zhang X, Tieu BC, Choudhary S, Brasier AR, Denner LA, Tilton RG (2006) Diabetes-induced activation of canonical and noncanonical nuclear factor-kappaB pathways in renal cortex. Diabetes 55(5):1252–1259. https://doi.org/10.2337/db05-1554
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. https://doi.org/10.1101/cshperspect.a001651
Mulero MC, Huxford T, Ghosh G (2019) NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. Adv Exp Med Biol 1172:207–226. https://doi.org/10.1007/978-981-13-9367-9_10
Chau TL, Gioia R, Gatot JS, Patrascu F, Carpentier I, Chapelle JP, O’Neill L, Beyaert R, Piette J, Chariot A (2008) Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem Sci 33(4):171–180. https://doi.org/10.1016/j.tibs.2008.01.002
Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M (1997) The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell 91(2):243–252. https://doi.org/10.1016/s0092-8674(00)80406-7
Rothwarf DM, Zandi E, Natoli G, Karin M (1998) IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395(6699):297–300. https://doi.org/10.1038/26261
Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, Matsumoto K (2000) TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 5(4):649–658. https://doi.org/10.1016/s1097-2765(00)80244-0
Hirata Y, Takahashi M, Morishita T, Noguchi T, Matsuzawa A (2017) Post-translational modifications of the TAK1-TAB complex. Int J Mol Sci 18 (1). doi:https://doi.org/10.3390/ijms18010205
Shi M, Deng W, Bi E, Mao K, Ji Y, Lin G, Wu X, Tao Z, Li Z, Cai X, Sun S, Xiang C, Sun B (2008) TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat Immunol 9(4):369–377. https://doi.org/10.1038/ni1577
Qiu H, Huang F, Xiao H, Sun B, Yang R (2013) TRIM22 inhibits the TRAF6-stimulated NF-kappaB pathway by targeting TAB2 for degradation. Virol Sin 28(4):209–215. https://doi.org/10.1007/s12250-013-3343-4
Funding
This project was funded by Health Scientific project of Heilongjiang Province (2020–372).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
W. Quan, L. Wang contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quan, W., Wang, L. Tripartite motif containing 23 functions as a critical regulator in macrophages to control the pathological feature of diabetic nephropathy. Int Urol Nephrol 55, 1263–1270 (2023). https://doi.org/10.1007/s11255-022-03419-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03419-7