Abstract
Purpose
To predict the efficacy of intravesical BCG therapy in patients with nonmuscle-invasive bladder tumors (NIBC) by using components of the cellular immune response such as the tuberculin skin test (PPD) and natural killer (NK) activity measurement.
Methods
Ninety-nine patients who were started on intravesical BCG therapy for NIBC were evaluated prospectively. Patients who were included in the intermediate, high, and very high-risk groups according to the EAU NMIBC Scoring System and who had never received intravesical BCG therapy previously were included. The clinical and demographic characteristics of the patients (age, gender, EAU NMBIC risk group, EORTC progression and recurrence scores, CUETO progression and recurrence scores, presence and types of comorbidity) were recorded. NK activity was measured and the PPD test was applied 3 days before the start of intravesical BCG therapy. The results of PPD were measured in millimeters 72 h after the test.
Results
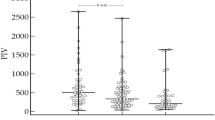
PPD values measured before BCG treatment, as an independent variable, were found to be significantly lower in patients with recurrence. A significant correlation was detected between NK activity results obtained before BCG treatment and recurrence after treatment, when the cutoff was 200–500 pg/dl. There was no significant relationship between the time to recurrence and PPD and NKA measurements.
Conclusion
We conclude that the results of PPD test and NK activity measurement performed before starting intravesical BCG therapy in NIBC may be a marker that can be used to predict the risk of recurrence under treatment.

Similar content being viewed by others
References
Burger M, Oosterlinck W, Konety B et al (2013) ICUD-EAU International Consultation on Bladder Cancer 2012: non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 63:36–44. https://doi.org/10.1016/j.eururo.2012.08.061
Fragkoulis C, Glykas I, Bamias A, Stathouros G, Papadopoulos G, Ntoumas K (2021) Novel treatments in BCG failure. Where do we stand today? Arch Esp Urol 74:681–691
Larsen ES, Joensen UN, Poulsen AM, Goletti D, Johansen IS (2020) Bacillus Calmette-Guerin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. APMIS 128:92–103. https://doi.org/10.1111/apm.13011
Fernandez-Gomez J, Madero R, Solsona E et al (2009) Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol 182:2195–2203. https://doi.org/10.1016/j.juro.2009.07.016
Shang PF, Kwong J, Wang ZP et al (2011) Intravesical Bacillus Calmette-Guerin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev 11(5):CD006885. https://doi.org/10.1002/14651858.CD006885.pub2 (PMID: 21563157)
Garcia-Cuesta EM, Esteso G, Ashiru O et al (2017) Characterization of a human anti-tumoral NK cell population expanded after BCG treatment of leukocytes. Oncoimmunology 6:e1293212. https://doi.org/10.1080/2162402X.2017.1293212 (PMID: 28507799; PMCID: PMC5414876)
Krpina K, Babarovic E, Dordevic G, Markic A, Jonjic N (2014) Impact of NK cell count on bladder cancer recurrence. Urologia 81:233–236. https://doi.org/10.5301/uro.5000063
Bryceson YT, Chiang SC, Darmanin S et al (2011) Molecular mechanisms of natural killer cell activation. J Innate Immun 3:216–226. https://doi.org/10.1159/000325265
Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K (2000) Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356:1795–1799. https://doi.org/10.1016/S0140-6736(00)03231-1
Hersey P, Edwards A, Honeyman M, McCarthy WH (1979) Low natural-killer-cell activity in familial melanoma patients and their relatives. Br J Cancer 40:113–122. https://doi.org/10.1038/bjc.1979.147
Pross HF, Sterns E, MacGillis DR (1984) Natural killer cell activity in women at “high risk” for breast cancer, with and without benign breast syndrome. Int J Cancer 34:303–308. https://doi.org/10.1002/ijc.2910340303
Schantz SP, Shillitoe EJ, Brown B, Campbell B (1986) Natural killer cell activity and head and neck cancer: a clinical assessment. J Natl Cancer Inst 77:869–875
Haggstrom C, Stocks T, Rapp K et al (2011) Metabolic syndrome and risk of bladder cancer:prospective cohort study in the metabolic syndrome and cancer project (Me-Can). Int J Cancer 128:1890–1398. https://doi.org/10.1002/ijc.25521
Newton CC, Gapstur SM, Campbell PT, Jacobs EJ (2013) Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer 132:2186–2191. https://doi.org/10.1002/ijc.27878
Krajewski W, Zdrojowy R, Grzegolka J et al (2019) Does mantoux test result predicts BCGimmunotherapy efficiency and severe toxicity in non-muscle invasive bladder cancer. Urol J 16:458–462
Carballido J, Alvarez-Mon M, Solovera OJ, Menendez-Ondina L, Durantez A (1990) Clinicalsignificance of natural killer activity in patients with transitional cell carcinoma of the bladder. J Urol 143:29–33. https://doi.org/10.1016/s0022-5347(17)39854-3
Funding
None of the authors received support from any organization for the submitted work. No funding was received to assist with the preparation of this manuscript. No funding was received for conducting this study. No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
(1) GY: protocol/project development, data collection. (2) EG: protocol/project development. (3) MGK: manuscript writing/editing, data analysis. (4) BC: data collection, data analysis. (5) BA: data analysis, manuscript editing. (6) MO: data collection. (7) TG: data collection. (8) EO: protocol/project development, manuscript editing. All authors have made a significant contribution to the findings and methods in the paper. All authors have read and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. There is no conflicting interest.
Ethics approval
Approval was obtained from the ethics committee of Gaziosmanpasa Training and Research Hospital. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazici, G., Gokmen, E., Kose, M.G. et al. The use of natural killer cell activity and PPD test in the prediction of results in intravesical BCG treatment of patients with nonmuscle-invasive bladder cancer. Int Urol Nephrol 55, 301–308 (2023). https://doi.org/10.1007/s11255-022-03414-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03414-y