Abstract
Background
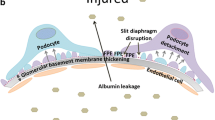
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease in the developed world. Podocyte injury is a critical cellular event involved in the progression of DN. Our previous studies demonstrated that platelet-derived microparticles (PMPs) mediated endothelial injury in diabetic rats. This study aimed to investigate whether PMPs are deposited in podocytes and to assess their potential effects on podocyte injury in DN.
Methods
The deposition of PMPs in podocytes was assessed by immunofluorescent staining and electron microscopy. The changes in renal pathology and ultra-microstructure were assessed by periodic acid-Schiff staining and electron microscopy, respectively. The expression of inflammatory cytokines and extracellular matrix proteins was measured by immuno-histochemical staining and western blot.
Results
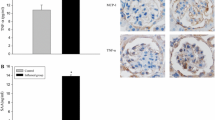
PMPs were widely deposited in podocytes of glomeruli in diabetic patients and animal models and closely associated with DN progression. Interestingly, aspirin treatment significantly inhibited the accumulation of PMPs in the glomeruli of diabetic rats, alleviated mesangial matrix expansion and fusion of foot processes, and decreased the protein expression of inflammatory cytokines and extracellular matrix secretion. An in vitro study further confirmed the deposition of PMPs in podocytes. Moreover, PMP stimulation induced the phenotypic transition of podocytes through decreased podocin protein expression and increased protein expression of α-SMA and fibronectin, which was correlated with increased production of inflammatory cytokines.
Conclusion
Our findings demonstrated for the first time that the deposition of PMPs in podocytes contributed to the development of DN.





Similar content being viewed by others
References
Umanath K, Lewis JB (2018) Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis 71(6):884–895. https://doi.org/10.1053/j.ajkd.2017.10.026
Ma RCW (2018) Epidemiology of diabetes and diabetic complications in China. Diabetologia 61(6):1249–1260. https://doi.org/10.1007/s00125-018-4557-7
Zhou Y, Echouffo-Tcheugui JB, Jian-jun Gu, Ruan X-N, Zhao G-M, Wang-hong Xu et al (2013) Prevalence of chronic kidney disease across levels of glycemia among adults in Pudong New Area, Shanghai China. BMC Nephrol 14:253
Jia W, Gao X, Pang C, Hou X, Bao Y, Liu W et al (2009) Prevalence and risk factors of albuminuria and chronic kidney disease in Chinese population with type 2 diabetes and impaired glucose regulation: Shanghai diabetic complications study (SHDCS). Nephrol Dial Transplant 24(12):3724–3731. https://doi.org/10.1093/ndt/gfp349
Lu B, Wen J, Song XY, Dong XH, Yang YH, Zhang ZY et al (2007) High prevalence of albuminuria in population-based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract 75(2):184–192. https://doi.org/10.1016/j.diabres.2006.06.024
Tang SCW, Yiu WH (2020) Innate immunity in diabetic kidney disease. Nat Rev Nephrol 16(4):206–222. https://doi.org/10.1038/s41581-019-0234-4
Hickey FB, Martin F (2018) Role of the immune system in diabetic kidney disease. Curr Diab Rep 18(4):20. https://doi.org/10.1007/s11892-018-0984-6
Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM et al (2019) A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 25(5):805–813. https://doi.org/10.1038/s41591-019-0415-5
Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L et al (2015) Inflammatory stress exacerbates lipid accumulation and podocyte injuries in diabetic nephropathy. Acta Diabetol 52(6):1045–1056. https://doi.org/10.1007/s00592-015-0753-9
Kao CY, Papoutsakis ET (2019) Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr Opin Biotechnol 60:89–98. https://doi.org/10.1016/j.copbio.2019.01.005
Kailashiya J, Gupta V, Dash D (2019) Engineered human platelet-derived microparticles as natural vectors for targeted drug delivery. Oncotarget 10(56):5835–5846
Ratajczak MZ, Ratajczak J (2020) Extracellular microvesicles/exosomes: discovery, disbelief, acceptance, and the future? Leukemia. https://doi.org/10.1038/s41375-020-01041-z
Akbar N, Azzimato V, Choudhury RP, Aouadi M (2019) Extracellular vesicles in metabolic disease. Diabetologia 62(12):2179–2187. https://doi.org/10.1007/s00125-019-05014-5
Melki I, Tessandier N, Zufferey A, Boilard E (2017) Platelet microvesicles in health and disease. Platelets 28(3):214–221. https://doi.org/10.1080/09537104.2016.1265924
Pardo F, Villalobos-Labra R, Sobrevia B, Toledo F, Sobrevia L (2018) Extracellular vesicles in obesity and diabetes mellitus. Mol Aspects Med 60:81–91. https://doi.org/10.1016/j.mam.2017.11.010
Hooten NN, Evans MK (2020) Extracellular vesicles as signaling mediators in type 2 diabetes mellitus. Am J Physiol Cell Physiol 3186(6):C1189–C1199. https://doi.org/10.1152/ajpcell.00536.2019
Li S, Wei J, Zhang C, Li X, Meng W, Mo X et al (2016) Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell Physiol Biochem 39(6):2439–2450. https://doi.org/10.1159/000452512
Agouni A, Andriantsitohaina R, Martinez M (2014) Microparticles as biomarkers of vascular dysfunction in metabolic syndrome and its individual components. Curr Vasc Pharmacol 12(3):483–492. https://doi.org/10.2174/1570161112666140423223148
Zhang Y, Ma KL, Gong YX, Wang GH, Hu ZB, Liu L et al (2018) Platelet microparticles mediate glomerular endothelial injury in early diabetic nephropathy. J Am Soc Nephrol 29(11):2671–2695. https://doi.org/10.1681/ASN.2018040368
Zhang C, Ou Q, Gu Y, Cheng G, Du R, Yuan L et al (2019) Circulating tissue factor-positive procoagulant microparticles in patients with type 1 diabetes. Diabetes Metab Syndr Obes 12:2819–2828. https://doi.org/10.2147/DMSO.S225761
Gkaliagkousi E, Nikolaidou B, Gavriilaki E, Lazaridis A, Yiannaki E, Anyfanti P et al (2019) Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diab Vasc Dis Res 16(5):458–465. https://doi.org/10.1177/1479164119844691
Stepanian A, Bourguignat L, Hennou S, Coupaye M, Hajage D, Salomon L et al (2013) Microparticle increase in severe obesity: not related to metabolic syndrome and unchanged after massive weight loss. Obesity (Silver Spring) 21(11):2236–2243. https://doi.org/10.1002/oby.20365
Santilli F, Marchisio M, Lanuti P, Boccatonda A, Miscia S, Davi G (2016) Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost 116(2):220–234. https://doi.org/10.1160/TH16-03-0176
Wang GH, Ma KL, Zhang Y, Hu ZB, Liu L, Lu J et al (2019) Platelet microparticles contribute to aortic vascular endothelial injury in diabetes via the mTORC1 pathway. Acta Pharmacol Sin 40(4):468–476. https://doi.org/10.1038/s41401-018-0186-4
Benameur T, Osman A, Parray A, Ait Hssain A, Munusamy S, Agouni A (2019) Molecular mechanisms underpinning microparticle-mediated cellular injury in cardiovascular complications associated with diabetes. Oxid Med Cell Longev 2019:6475187. https://doi.org/10.1155/2019/6475187
Rodrigues KF, Pietrani NT, Fernandes AP, Bosco AA, de Sousa MCR, de Fátima OliveiraSilva I et al (2018) Circulating microparticles levels are increased in patients with diabetic kidney disease: a case-control research. Clin Chim Acta 479:48–55. https://doi.org/10.1016/j.cca.2017.12.048
Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L et al (2013) The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med 5(2):235–249. https://doi.org/10.1002/emmm.201201846
Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME et al (2010) Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327(5965):580–583. https://doi.org/10.1126/science.1181928
Teoh NC, Ajamieh H, Wong HJ, Croft K, Mori T, Allison AC et al (2014) Microparticles mediate hepatic ischemia-reperfusion injury and are the targets of diannexin (ASP8597). PLoS ONE 9(9):e104376. https://doi.org/10.1371/journal.pone.0104376
Barry OP, Praticò D, Savani RC, FitzGerald GA (1998) Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest 102(1):136–144. https://doi.org/10.1172/JCI2592
Qu M, Zou X, Fang F, Wang S, Xu L, Zeng Q et al (2020) Platelet-derived microparticles enhance megakaryocyte differentiation and platelet generation via miR-1915–3p. Nat Commun. https://doi.org/10.1038/s41467-020-18802-0
Liang C, Huang J, Luo P, Wang Z, He J, Wu S et al (2020) Platelet-derived microparticles mediate the intra-articular homing of mesenchymal stem cells in early-stage cartilage lesions. Stem Cells Dev 29(7):414–424. https://doi.org/10.1089/scd.2019.0137
Plantureux L, Mege D, Crescence L, Carminita E, Robert S, Cointe S et al (2020) The interaction of platelets with colorectal cancer cells inhibits tumor growth but promotes metastasis. Cancer Res 80(2):291–303. https://doi.org/10.1158/0008-5472.CAN-19-1181
Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E et al (2013) Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood 122(2):253–261. https://doi.org/10.1182/blood-2013-03-492801
Michael JV, Wurtzel JGT, Mao GF, Rao AK, Kolpakov MA, Sabri A et al (2017) Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood 130(5):567–580. https://doi.org/10.1182/blood-2016-11-751099
Kyselova A, Elgheznawy A, Wittig I, Heidler J, Mann AW, Ruf W et al (2020) Platelet-derived calpain cleaves the endothelial protease-activated receptor 1 to induce vascular inflammation in diabetes. Basic Res Cardiol 115(6):75. https://doi.org/10.1007/s00395-020-00833-9
Olumuyiwa-Akeredolu OO, Page MJ, Soma P, Pretorius E (2019) Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat Rev Rheumatol 15(4):237–248. https://doi.org/10.1038/s41584-019-0187-9
Giacomazzi A, Degan M, Calabria S, Meneguzzi A, Minuz P (2016) Antiplatelet agents inhibit the generation of platelet-derived microparticles. Front Pharmacol 7:314. https://doi.org/10.3389/fphar.2016.00314
Chen Y, Xiao Y, Lin Z, Xiao X, He C, Bihl JC et al (2015) The role of circulating platelets microparticles and platelet parameters in acute ischemic stroke patients. J Stroke Cerebrovasc Dis 24(10):2313–2320. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.06.018
Cheng G, Shan XF, Wang XL, Dong WW, Li Z, Liu XH et al (2017) Endothelial damage effects of circulating microparticles from patients with stable angina are reduced by aspirin through ERK/p38 MAPKs pathways. Cardiovasc Ther 35(4):e12273. https://doi.org/10.1111/1755-5922.12273
Acknowledgements
We are grateful to all participants for their efforts.
Funding
This study was supported by the National Natural Science Foundation of China (82170736, 81970629), the Project for Jiangsu Provincial Medical Talent (ZDRCA2016077), the Fundamental Research Funds for the Central Universities (3224002110D), and the Jiangsu Province Ordinary University Graduate Research Innovation Project (SJCX20-0055).
Author information
Authors and Affiliations
Contributions
KLM: designed the study. SJH, YZ, GHW, JL, PPC, JXZ, and XQL: performed the experiments and established the genetically modified mouse models. BYY, LQL, TTJ, MYW and WTL: analyzed the data. XZR and BCL: drafted and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Zhongda Hospital, Affiliated to Southeast University (No. 2016ZDSYLL003.0).
Informed consent
All patients signed the written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, S.J., Zhang, Y., Wang, G.H. et al. Deposition of platelet-derived microparticles in podocytes contributes to diabetic nephropathy. Int Urol Nephrol 55, 355–366 (2023). https://doi.org/10.1007/s11255-022-03332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03332-z