Abstract
Purpose
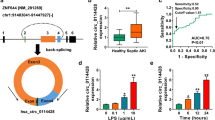
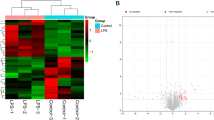
Sepsis is a systemic life-threatening inflammatory disease, which leads to septic acute kidney injury (AKI). Circular RNAs (circRNAs) are involved in septic AKI. Herein, we aimed to expound the action of circ_0020339 in septic AKI. The dysregulation of plasma circRNAs between patients with septic non-AKI and patients with septic AKI were screened by circRNA chip.
Methods
The dysregulation of circ_0020339, microRNA (miR)-17-5p, and inositol polyphosphate multi kinase (IPMK) mRNA was detected by quantitative real-time polymerase chain reaction (qRT-PCR). Cell viability and apoptosis were measured by cell counting kit-8 (CCK-8) and flow cytometry, respectively. The release of serum creatinine (SCr), tissue inhibitor metalloproteinase-2 (TIMP-2), insulin-like growth factor binding protein-7 (IGFBP7), tumor necrosis factor (TNF)α and interleukin (IL)-1β was evaluated by enzyme-linked immunosorbent assay (ELISA). Bioinformatic analysis, dual-luciferase reporter assay and miRNA pull down assay were used to confirm the interaction between miR-17-5p and circ_0020339 or IPMK 3’untranslated region (UTR). Protein level of IPMK, TNF receptor-associated factor 6 (TRAF6), phosphorylated AKT (p-AKT)/total (t)-AKT, p-nuclear factor kappa-B (NF-κB) kinase (p-IKK)/t-IKK, p-inhibitor of NF-κB (p-IκB)α/t-IκBα, and p-p65/t-p65 were conducted by western blot.
Results
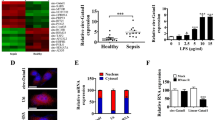
Circ_0020339 was upregulated in the plasma of patients with septic AKI as well as LPS-treated HK2 cells and C57BL/6 mice relative to the corresponding counterparts. Functionally, circ_0020339 was positively correlated with markers of renal functional injury and inflammation in patients with septic AKI; si-circ_0020339 facilitated cell proliferation, while restrained cell apoptosis and inflammation in LPS-triggered HK2 cells; meanwhile, si-circ_0020339 restrained survival rate, renal functional injury and inflammation in LPS-triggered C57BL/6 mice. Furthermore, circ_0020339 and IPMK 3’UTR shared the same complementary sites with miR-17-5p.
Conclusion
si-circ_0020339 attenuated LPS-induced cell damage by targeting miR-17-5p/IPMK axis and inactivation of TRAF6/p-AKT/p-IKK/p-IκBα/p-p65. Altogether, plasma circ_0020339 serves as a novel diagnostic marker of patients with septic AKI.







Similar content being viewed by others
Availability of data and material
They are available from the corresponding author on special request.
References
Cecconi M, Evans L, Levy M, Rhodes A (2018) Sepsis and septic shock. Lancet 392:75–87. https://doi.org/10.1016/S0140-6736(18)30696-2
Poston JT, Koyner JL (2019) Sepsis associated acute kidney injury. BMJ 364:4891. https://doi.org/10.1136/bmj.k4891
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818. https://doi.org/10.1001/jama.294.7.813
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J (2019) The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 20:675–691. https://doi.org/10.1038/s41576-019-0158-7
Beltran-Garcia J, Osca-Verdegal R, Nacher-Sendra E, Pallardo FV, Garcia-Gimenez JL (2020) Circular RNAs in sepsis: biogenesis, function, and clinical significance. Cells 9:1544. https://doi.org/10.3390/cells9061544
Brandenburger T, Salgado Somoza A, Devaux Y, Lorenzen JM (2018) Noncoding RNAs in acute kidney injury. Kidney Int 94:870–881. https://doi.org/10.1016/j.kint.2018.06.033
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19:141–157. https://doi.org/10.1261/rna.035667.112
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854. https://doi.org/10.1016/0092-8674(93)90529-y
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11:597–610. https://doi.org/10.1038/nrg2843
Colbert JF, Ford JA, Haeger SM, Yang Y, Dailey KL, Allison KC, Neudecker V, Evans CM, Richardson VL, Brodsky KS, Faubel S, Eltzschig HK, Schmidt EP, Ginde AA (2017) A model-specific role of microRNA-223 as a mediator of kidney injury during experimental sepsis. Am J Physiol Renal Physiol 313:F553–F559. https://doi.org/10.1152/ajprenal.00493.2016
Ma J, Li Y, Zhang S, Fu S, Ye X (2019) MiR-590–3p attenuates acute kidney injury by inhibiting tumor necrosis factor receptor-associated factor 6 in septic mice. Inflammation 42:637–649. https://doi.org/10.1007/s10753-018-0921-5
Guan Y, Ma J, Song W (2019) Identification of circRNA-miRNA-mRNA regulatory network in gastric cancer by analysis of microarray data. Cancer Cell Int 19:183. https://doi.org/10.1186/s12935-019-0905-z
Wong CH, Lou UK, Li Y, Chan SL, Tong JH, To K, Chen Y (2020) CircFOXK2 promotes growth and metastasis of pancreatic ductal adenocarcinoma by complexing with RNA binding proteins and sponging MiR-942. Cancer Res 80:2138–2149. https://doi.org/10.1158/0008-5472.CAN-19-3268
Li H, Zhang X, Wang P, Dai J, Li Z, Liu B, Li H, Ai K, Zheng P, Qiu S, Li Y, Wang Y, Xiang X, Chai X, Dong Z, Zhang D (2021) Knockdown of circ-FANCA alleviates LPS-induced HK2 cell injury via targeting miR-93–5p/OXSR1 axis in septic acute kidney injury. Diabetol Metab Syndr 13:7. https://doi.org/10.1186/s13098-021-00625-8
Tan M, Bei R (2021) Circ_0091702 serves as a sponge of miR-545–3p to attenuate sepsis-related acute kidney injury by upregulating THBS2. J Mol Histol 52:717–728. https://doi.org/10.1007/s10735-021-09991-z
He Y, Sun Y, Peng J (2021) Circ_0114428 regulates sepsis-induced kidney injury by targeting the miR-495–3p/CRBN axis. Inflammation 44:1464–1477. https://doi.org/10.1007/s10753-021-01432-z
Rhodes A, Evans LE, Alhazzani W et al (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552. https://doi.org/10.1097/CCM.0000000000002255
Drüeke TB, Parfrey PS (2012) Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int 82:952–960. https://doi.org/10.1038/ki.2012.270
Beutler B, Rietschel ET (2003) Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 3:169–176. https://doi.org/10.1038/nri1004
Lameire N, Vanholder R, Van Biesen W, Benoit D (2016) Acute kidney injury in critically ill cancer patients: an update. Crit Care 20:209. https://doi.org/10.1186/s13054-016-1382-6
Stoyanoff TR, Todaro JS, Aguirre MV, Zimmermann MC, Brandan NC (2014) Amelioration of lipopolysaccharide-induced acute kidney injury by erythropoietin: involvement of mitochondria-regulated apoptosis. Toxicology 318:13–21. https://doi.org/10.1016/j.tox.2014.01.011
Meurer M, Höcherl K (2019) Deregulated renal magnesium transport during lipopolysaccharide-induced acute kidney injury in mice. Pflugers Arch 471:619–631. https://doi.org/10.1007/s00424-019-02261-8
Zhang P, Yi L, Qu S, Dai J, Li X, Liu B, Li H, Ai K, Zheng P, Qiu S, Li Y, Wang Y, Xiang X, Chai X, Dong Z, Zhang D (2020) The biomarker TCONS_00016233 drives septic AKI by targeting the miR-22–3p/AIFM1 signaling axis. Mol Ther Nucleic Acids 19:1027–1042. https://doi.org/10.1016/j.omtn.2019.12.037
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PcR and the 2(-delta deltac(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Wang K, Xie S, Xiao K, Yan P, He W, Xie L (2018) Biomarkers of sepsis-induced acute kidney injury. BioMed Res Int 2018:6937947. https://doi.org/10.1155/2018/6937947
Morrell ED, Kellum JA, Pastor-Soler NM, Hallows KR (2014) Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Crit Care 18:501. https://doi.org/10.1186/s13054-014-0501-5
Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C, Acute Dialysis Quality Initiative Consensus XIII Work Group (2016) Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27:371–379. https://doi.org/10.1681/ASN.2015030261
Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P (2020) Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-kappaB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother 122:109772. https://doi.org/10.1016/j.biopha.2019.109772
Liu Z, Tang C, He L, Yang D, Cai J, Zhu J, Shu S, Liu Y, Yin L, Chen G, Liu Y, Zhang D, Dong Z (2020) The negative feedback loop of NF-kappaB/miR-376b/NFKBIZ in septic acute kidney injury. JCI Insight 5:e142272. https://doi.org/10.1172/jci.insight.142272
Kim E, Beon J, Lee S, Park SJ, Ahn H, Kim MG, Park JE, Kim W, Yuk J, Kang S, Lee S, Jo E, Seong J, Lee S, Jo E, Seong RH, Kim S (2017) Inositol polyphosphate multi kinase promotes Toll-like receptor-induced inflammation by stabilizing TRAF6. Sci Adv 3:e1602296. https://doi.org/10.1126/sciadv.1602296
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen Z (2000) Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351–361. https://doi.org/10.1016/s0092-8674(00)00126-4
Maag D, Maxwell MJ, Hardesty DA, Boucher KL, Choudhari N, Hanno AG, Ma JF, Snowman AS, Pietropaoli JW, Xu R, Storm PB, Saiardi A, Snyder SH, Resnick AC (2011) Inositol polyphosphate multi kinase is a physiologic PI3-kinase that activates Akt/PKB. Proc Natl Acad Sci USA 108:1391–1396. https://doi.org/10.1073/pnas.1017831108
Anders HJ, Banas B, Schlöndorff D (2004) Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15:854–867. https://doi.org/10.1097/01.asn.0000121781.89599.16
Yuan WS, Xiong XQ, Du JL, Fan Q, Wang R, Zhang X (2021) LncRNA PVT1 accelerates LPS-induced septic acute kidney injury through targeting miR-17–5p and regulating NF-κB pathway. Int Urol Nephrol 53:2409–2419. https://doi.org/10.1007/s11255-021-02905-8
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. LW, BB, DZ, and ZW conducted the experiments, DX wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Current study was approved by the Ethics Committee of People’s Hospital of Xinjiang Uygur Autonomous Region.
Consent to participate
All patients have provided the written informed consent prior to the start of the study.
Consent for publication
All patients have agreed to share their clinical data for scientific research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Bayinchahan, B., Zhang, D. et al. The novel biomarker circ_0020339 drives septic acute kidney injury by targeting miR-17-5p/IPMK axis. Int Urol Nephrol 55, 437–448 (2023). https://doi.org/10.1007/s11255-022-03331-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03331-0