Abstract
Purpose
In this study we aimed to screen for the presence of biomarkers that are downregulated in children with nephrolithiasis (RS) compared to healthy controls (HC) using a proteomic approach. We hypothesized that RS and HC would display unique inhibitory protein profiles that could be used for comparative pathway analysis.
Methods
This is a prospective, controlled, pilot study of pooled urine from RS (N = 30, 24 females, mean age 12.95 ± 4.03 years) versus age- and gender-matched HC, using liquid chromatography-mass spectrometry. The criteria for protein selection were: (1) patient/control abundance ratio of < 0.5; and (2) ≤ 0.05 p-value for the Fisher’s Exact Test. Results were confirmed by ELISA testing in individual samples.
Results
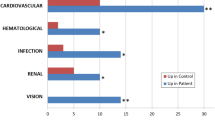
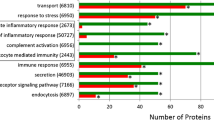
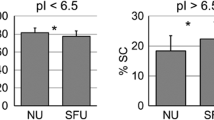
67 proteins were downregulated in RS group, and 17 of those were significantly different compared to controls. Of those seventeen proteins, five (two actins, annexin A5, keratin 6B, and serpin B4) were completely absent in the urine of stone patients but were found in controls. The remaining twelve proteins were significantly less abundant in the patient’s urine compared to healthy controls. Protein–protein interaction modeling of significant proteins identified syndecan-1 as the key node, a protein associated with adhesion pathways. ELISA analysis by subgroups showed statistically significant difference in the urinary excretion of osteopontin (5.1 ± 3.22 ng/mg creatinine vs 14.1 ± 9.5 ng/mg creatinine, p = 0.046) between stone patients with hypocitraturia and controls. Urinary osteopontin concentration was positively correlated with urinary citrate excretion (r = 0.417, p = 0.03).
Conclusions
Children with RS have a different urinary inhibitory polypeptide profile compared to HC. Decreased urinary excretion of these proteins indicates their potential inhibitory role in renal stone formation, especially of the adhesion phase. Lower concentration of urinary osteopontin in children with nephrolithiasis and hypocitraturia suggests its potential involvement in the pathogenesis of this disease. Further characterization of these proteins in a larger sample is imperative.

Similar content being viewed by others
References
Coe FL, Nakagawa Y, Asplin J et al (1994) Role of nephrocalcin in inhibition of calcium oxalate crystallization and nephrolithiasis. Miner Electrolyte Metab 20:378–384
Hess B (1994) Tamm-Horsfall glycoprotein and calcium nephrolithiasis. Miner Electrolyte Metab 20:393–398
Boyce WH (1968) Organic matrix of human urinary concretions. Am J Med 45(5):673–683
Adachi J, Kumar C, Zhang Y (2006) The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol 7(9):R80
Kovacevic L, Lu H, Kovacevic N, Thomas R, Lakshmanan Y (2020) Urinary cystatin C, neutrophil gelatinase-associated lipocalin, and lysozyme C: novel biomarkers for detection of early kidney dysfunction in children with urolithiasis. Urology 143:221–226
Thongboonkerd V (2007) Practical points in urinary proteomics. J Proteome Res 6(10):3881–3890
Neilson KA, Ali NA, Muralidharan S et al (2011) Less label, more free: approaches in label-free quantitative mass spectrometry. Proteomics 11:535–553
Healy KA, Hubosky SG, Bagley DH (2013) 24-hour urine collection in the metabolic evaluation of stone formers: is one study adequate? J Endourol 27(3):374–378
Nayan M, Elkoushy MA, Andonian S (2012) Variations between two 24-hour urine collections in patients presenting to a tertiary stone clinic. Can Urol Assoc J 6(1):30–33
Cameron MA, Sakhaee K, Moe OW (2005) Nephrolithiasis in children. Pediatr Nephrol 20:1587–1592
Hoppe B, Kemper MJ (2010) Diagnostic examination of the child with urolithiasis or nephrocalcinosis. Pediatr Nephrol 25:403–413
Chutipongtanate S, Nakagawa Y, Sritippayawan S et al (2005) Identification of human urinary trefoil factor 1 as a novel calcium oxalate crystal growth inhibitor. J Clin Invest Actions 115(12):3613–3622
Wesson JA, Johnson RJ, Mazzali M et al (2003) Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol 14(1):139–147
Kumar V, Lieske JC (2006) Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens 15(4):374–380
Suzuki K, Ryall RL, Suzuki K et al (1996) The effect of heparan sulphate on the crystallization of calcium oxalate in undiluted, ultrafiltered human urine. Br J Urol 78(1):15–21
Yamaguchi S, Yoshioka T, Utsunomiya M et al (1993) Heparin sulfate in the stone matrix and its inhibitory effect on calcium oxalate crystallization. Urol Res 21(3):187–192
Shum DKY, Gohel MDI (1993) Separate effects of urinary chondroitin sulfate and heparin sulfate upon the crystallization of urinary calcium oxalate: differences between stone formers and normal controls. Clin Sci 85:33–39
Suzuki K, Ryall RL (1996) The effect of hep[aran sulfate on the crystallization of calcium oxalatein undiluted, ultrafiltered human urine. Br J Urol 78(1):15–21
Chikama S, Iida S, Inoue M et al (2002) Role of heparan sulfate proteoglycan (syndecan-1) on the renal epithelial cells during calcium oxalate monohydrate crystal attachment. Kurume Med J 49(4):201–210
Yoneyama AG, Tobisawa ND, Hatakeyama S et al (2019) The impact of glycosylation of osteopontin on urinary stone formation. Int J Mol Sci 21(1):93
Kazanecki CC, Uzwiak DJ, Denhardt DT (2007) Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem 102:912–924
Sodek J, Ganss B, McKee M (2000) Osteopontin. Crit Rev Oral Biol Med 11:279–303
Evan AP, Coe FL, Rittling SR (2005) Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int 68(1):145–154
Khan SR, Kok DJ (2004) Modulators of urinary stone formation. Front Biosci 9:1450–1482
Ryall RL, Ryall RL (1996) Glycosaminoglycans, proteins, and stone formation: adult themes and child’s play. Pediatr Nephrol 10(5):656–66
Li X, Liu K, Pan Y et al (2015) Roles of osteopontin gene polymorphism (rs1126616), osteopontin levels in urine and serum, and the risk of urolithiasis: a meta-analysis. Biomed Res Int Actions 2015:315043
JR Hoyer, Otvos L Jr, Urge L (1995) Osteopontin in urinary stone formation. Ann NY Acad Sci 760:257–265
Wright CA, Howles S, Trudgian DC et al (2011) Label-free quantitative proteomics reveals differentially regulated proteins influencing urolithiasis. Mol Cell Proteomics 10(8):M110.005686
Cadieux PA, Beiko DT, Watterson JD et al (2004) Surface-enhanced laser desorption/ionization-time of flight-mass spectrometry (SELDI-TOF-MS): a new proteomic urinary test for patients with urolithiasis. J Clin Lab Anal 18(3):170–175
Bautista DS, Denstedt J, Chambers AF et al (1996) Low-molecular-weight variants of osteopontin generated by serine proteinases in urine of patients with kidney stones. J Cell Biochem 61(3):402–409
Beshensky WJA, Worcester EM et al (2001) Effects of urinary macromolecules on hydroxyapatite crystal formation. J Am Soc Nephrol 12:2108–2116
Asplin JR, Assenault D, Parks JH et al (1998) Contribution of human uropontin to inhibition of calcium oxalate crystallization. Kidney Int 53:194–199
Qiu SR, Wierzbicki A, Orme CA (2004) Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc Natl Acad Sci 101(7):1811–1815
Kovacevic L, Wolfe-Christensen C et al (2012) From hypercalciuria to hypocitraturia–a shifting trend in pediatric urolithiasis? J Urol 188(4):1623–1627
Funding
This study was funded by Children’s Hospital of Michigan Foundation (grant number R2-2014-31).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Larisa Kovacevic declares that she has no conflict of interest. Author Natalija Kovacevic declares that she has no conflict of interest. Author Yegappan Lakshmanan declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kovacevic, L., Kovacevic, N. & Lakshmanan, Y. Proteomic analysis of inhibitory protein profiles in the urine of children with nephrolithiasis: implication for disease prevention. Int Urol Nephrol 54, 2783–2788 (2022). https://doi.org/10.1007/s11255-022-03310-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03310-5