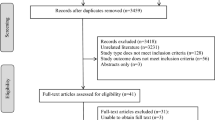
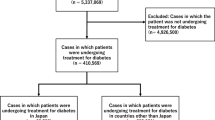
Abstract
Purpose
The sodium–glucose cotransporter-2 (SGLT2) inhibitors have changed the treatment of type 2 diabetes mellitus. Several studies evaluated SGLT2 inhibitor-related acute kidney injury (AKI), but pharmacoepidemiology studies are needed to compare the adverse events in different SGLT2 inhibitors (SGLT2i).
Methods
We used disproportionality analysis and Bayesian analysis in data mining to screen the AKI cases after initiating different SGLT2i among diabetic patients, based on the FDA’s Adverse Event Reporting System (FAERS) updated to December 2020. We also investigated the onset time and fatality rates of SGLT2i-associated AKI, which was based on preferred terms (PTs) coded for the renal adverse events in the structure of the FARES database.
Results
We identified 2483 cases of AKI following SGLT2i regimens among diabetic patients. Most of them were 45–64 years old (58.46%) and > 65 years old (28.67%). Canagliflozin generated the largest number of AKI reports (n = 1650, 66.45%) in our study. Canagliflozin showed the strongest association among SGLT2i, evidenced by the highest reporting odds ratio (ROR = 3.70, two-sided 95% CI 3.51–3.91), proportional reporting ratio (PRR = 3.39, χ2 = 2635.06), and empirical Bayes geometric mean (EBGM = 3.18, one-sided 95% CI 3.04). The median onset time to AKI was 72.0 (interquartile range [IQR] 21.0–266.0) days after SGLT2i initiation. The general hospitalization rate of SGLT2i-associated AKI was 63.50%, and the fatality rate was 1.59%. The deceased patients (62.94 ± 10.69 years) were significantly older than the survived ones (57.82 ± 11.84 years) (P = 0.011).
Conclusion
We compared AKI events in the real-world practice of various SGLT2i among diabetic cases from the FAERS database. It is essential to monitor kidney function during the early administration of SGLT2i. Concern should be paid for AKI in patients older than 65 taking SGLT2i.


Similar content being viewed by others
Availability of data and materials
All necessary data have been presented as tables and figures in the manuscript. Related information is accessible under request to the corresponding author.
Abbreviations
- AKI:
-
Acute kidney injury
- SGLT2:
-
Sodium–glucose cotransporter-2
- SGLT2i:
-
SGLT2 inhibitor
- AE:
-
Adverse event
- FAERS:
-
The Food and Drug Administration’s Adverse Event Reporting System
- ROR:
-
Reporting odds ratio
- PRR:
-
Proportional reporting ratio
- EBGM:
-
Empirical Bayes geometric mean
- IQR:
-
Interquartile range
- FDA:
-
The Food and Drug Administration
- SRS:
-
Spontaneous reporting system
- BCPNN:
-
Bayesian confidence propagation neural network
- MGPS:
-
Multi-item gamma Poisson shrinker
- RAS:
-
Renin–angiotensin system
References
Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med 66:255–270
DeFronzo RA, Norton L, Abdul-Ghani M (2017) Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol 13:11–26
Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, von Eynatten M (2016) The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 59:1860–1870
Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjöström CD (2016) Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia 59:2036–2039
Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L (2018) Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 20:458–462
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB (2014) Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 8:262–75.e9
Muskiet MHA, van Bommel EJ, van Raalte DH (2016) Antihypertensive effects of SGLT2 inhibitors in type 2 diabetes. Lancet Diabetes Endocrinol 4:188–189
Cefalu WT, Stenlöf K, Leiter LA, Wilding JP, Blonde L, Polidori D, Xie J, Sullivan D, Usiskin K, Canovatchel W, Meininger G (2015) Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia 58:1183–1187
Verma S, McMurray JJV (2018) SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 61:2108–2117
Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ (2016) Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol 12:711–712
Shrivastava S, Srivastava N, Alfanso-Jaume M (2019) Acute renal failure with cocaine and SGLT-2 inhibitor. Am J Ther 26:e762–e763
Phadke G, Kaushal A, Tolan DR, Hahn K, Jensen T, Bjornstad P, Roncal-Jimenez C, Hernando AA, Lanaspa MA, Alexander MP, Kukla A, Johnson RJ (2020) Osmotic nephrosis and acute kidney injury associated with SGLT2 inhibitor use: a case report. Am J Kidney Dis 76:144–147
Usiskin K, Kline I, Fung A, Mayer C, Meininger G (2014) Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: pooled analysis of phase 3 study results. Postgrad Med 126:16–34
Darawshi S, Yaseen H, Gorelik Y, Faor C, Szalat A, Abassi Z, Heyman SN, Khamaisi M (2020) Biomarker evidence for distal tubular damage but cortical sparing in hospitalized diabetic patients with acute kidney injury (AKI) while on SGLT2 inhibitors. Ren Fail 42:836–844
Tang H, Li D, Zhang J, Li Y, Wang T, Zhai S, Song Y (2017) Sodium-glucose co-transporter-2 inhibitors and risk of adverse renal outcomes among patients with type 2 diabetes: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab 19:1106–1115
Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A (2013) Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 159:262–274
FDA. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). Available from: https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm. 2016.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Investigators CT (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380:2295–2306
Raschi E, Parisotto M, Forcesi E, La Placa M, Marchesini G, De Ponti F, Poluzzi E (2017) Adverse events with sodium-glucose co-transporter-2 inhibitors: a global analysis of international spontaneous reporting systems. Nutr Metab Cardiovasc Dis 27:1098–1107
Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A (2017) Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis 27:1108–1113
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380:347–357
Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, Laverman GD, Lim SK, Di Tanna GL, Reich HN, Vervloet MG, Wong MG, Gansevoort RT, Heerspink HJL (2020) Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 8:582–593
Menne J, Dumann E, Haller H, Schmidt BMW (2019) Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med 16:e1002983
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375:323–334
Sridhar VS, Tuttle KR, Cherney DZI (2020) We can finally stop worrying about SGLT2 inhibitors and acute kidney injury. Am J Kidney Dis 76:454–456
Desai M, Yavin Y, Balis D, Sun D, Xie J, Canovatchel W, Rosenthal N (2017) Renal safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus. Diabetes Obes Metab 19:897–900
Tobe SW, Clase CM, Gao P, McQueen M, Grosshennig A, Wang X, Teo KK, Yusuf S, Mann JF (2011) Cardiovascular and renal outcomes with telmisartan, ramipril, or both in people at high renal risk: results from the ONTARGET and TRANSCEND studies. Circulation 123:1098–1107
Garcia-Ropero A, Badimon JJ, Santos-Gallego CG (2018) The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: the latest developments. Expert Opin Drug Metab Toxicol 14:1287–1302
O’Sullivan ED, Hughes J, Ferenbach DA (2017) Renal aging: causes and consequences. J Am Soc Nephrol 28:407–420
Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E (2021) Existence of notoriety bias in FDA adverse event reporting system database and its impact on signal strength. Hosp Pharm 56:152–158
Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP, Overstreet BM (2014) The Weber effect and the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. Drug Saf 37:283–294
Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, George JT, Green JB, Landray MJ, Baigent C, Wanner C (2018) The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 11:749–761
Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC (2020) Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35:274–282
Acknowledgements
This work has been made possible through an ISN Sister Renal Centers Grant.
Funding
This study was supported by Thrombocytopenia Funding from Yeehong School of Shenyang Pharmaceutical University (TCP funding).
Author information
Authors and Affiliations
Contributions
GC designed the study, analyzed and interpreted data, generated figures/tables, and drafted the manuscript. XL analyzed and interpreted data, and drafted the manuscript. QC contributed to manuscript drafting. BZ designed the study and directed the data mining in the FAERS database. QC, YZ, DM, and XL reviewed and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, G., Li, X., Cui, Q. et al. Acute kidney injury following SGLT2 inhibitors among diabetic patients: a pharmacovigilance study. Int Urol Nephrol 54, 2949–2957 (2022). https://doi.org/10.1007/s11255-022-03211-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03211-7