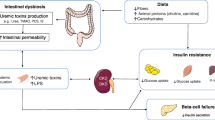
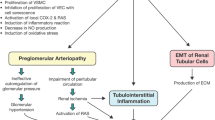
Abstract
Indoxyl sulphate (IS) a representative uraemic toxin in the blood of patients with chronic kidney disease (CKD). Its accumulation may be closely related to CKD and the increasing morbidity and mortality of the disease’s related complications. Timely and effective detection of the IS level and efficient clearance of IS may effectively prevent the progression of CKD and its related complications. Therefore, this article summarizes the research progress of IS related, including IS in CKD and its associated complications including chronic kidney disease, chronic kidney disease with cardiovascular disease, renal anemia, bone mineral metabolic disease and neuropsychiatric disorders, looking for IS accurate rapid detection methods, and explore the efficient treatment to reduce blood levels of indole phenol sulphate.


Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Jager KJ, Fraser SDS (2017) The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant 32(suppl_2):ii121–ii128
Bharati J, Jha V, Levin A (2021) The global kidney health Atlas: burden and opportunities to improve kidney health worldwide. Ann Nutr Metab 76(suppl1):1–6
Li HX, Lu WH, Wang A et al (2021) Changing epidemiology of chronic kidney disease as a result of type 2 diabetes mellitus from 1990 to 2017: estimates from global burden of disease 2017. J Diabetes Investig 12:346–356
Duan J, Wang C, Liu D et al (2019) Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in Chinese rural residents: a cross-sectional survey. Sci Rep 9(1):10408
Wang JW, Wang F, Saran R et al (2018) Mortality risk of chronic kidney disease: A comparison between the adult populations in urban China and the United States. PLoS ONE 13(3):e0193734
Hill NR, Fatoba ST, Oke JL et al (2016) Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS ONE 11(7):e0158765
Eloot S, Schneditz D, Cornelis T et al (2016) Protein-bound uremic toxin profiling as a tool to optimize hemodialysis. PLoS ONE 11(1):e0147159
Nigam Sanjay K, Wu W, Bush Kevin T et al (2015) Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10(11):2039–2049
Sirich Tammy L, Aronov Pavel A, Plummer Natalie S et al (2013) Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84(3):585–590
Snauwaert E, Holvoet E, Van Biesen W et al (2019) Uremic toxin concentrations are related to residual kidney function in the pediatric hemodialysis population. Toxins (Basel) 11(4):235
Xie T, Bao M, Zhang P et al (2019) Serum concentration of indoxyl sulfate in peritoneal dialysis patients and low-flux hemodialysis patients. Blood Purif 48(2):183–190
Holle J, Querfeld U, Kirchner M et al (2019) Indoxyl sulfate associates with cardiovascular phenotype in children with chronic kidney disease. Pediatr Nephrol 34(12):2571–2582
Adesso S, Popolo A, Bianco G et al (2013) The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS ONE 8(9):e76778
Ellis RJ, Small DM, Ng KL et al (2018) Indoxyl sulfate induces apoptosis and hypertrophy in human kidney proximal tubular cells. Toxicol Pathol 46(4):449–459
Yu Yan L, Xiao Dong L, Liu L et al (2016) Mechanism of indoxyl sulfate promoting renal fibrosis. J Third Mil Med Univ 38(02):119–123
Milanesi S, Garibaldi S, Saio M et al (2019) Indoxyl sulfate induces renal fibroblast activation through a targetable heat shock protein 90-dependent pathway. Oxid Med Cell Longev 2019:2050183
Dou L, Poitevin S, Sallée M et al (2018) Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int 93(4):986–999
Saito S, Yisireyili M, Shimizu H et al (2015) Indoxyl sulfate upregulates prorenin expression via nuclear factor-κB p65, signal transducer and activator of transcription 3, and reactive oxygen species in proximal tubular cells. J Ren Nutr 25(2):145–148
Kaushal GP, Chandrashekar K, Juncos LA (2019) Molecular interactions between reactive oxygen species and autophagy in kidney disease. Int J Mol Sci 20(15):3791
Edamatsu T, Fujieda A, Itoh Y (2018) Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PLoS ONE 13(2):e0193342
Li SZ, Cheng SJ, Sun ZZ et al (2016) Indoxyl sulfate induces mesangial cell proliferation via the induction of COX-2. Mediators Inflamm 2016:5802973
Major RW, Cheng MRI, Grant RA et al (2018) Cardiovascular disease risk factors in chronic kidney disease: a systematic review and meta-analysis. PLoS ONE 13(3):e0192895
Fan PC, Chang JC, Lin CN et al (2019) Serum indoxyl sulfate predicts adverse cardiovascular events in patients with chronic kidney disease. J Formos Med Assoc 118(7):1099–1106
Lin CJ, Wu V, Wu PC et al (2015) Meta-Analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 10(7):e0132589
Wang XR, Zhang JJ, Xu XX et al (2019) Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: a systematic review and meta-analysis. Ren Fail 41(1):244–256
Srivastava A, Kaze AD, McMullan CJ et al (2018) Uric acid and the risks of kidney failure and death in individuals with CKD. Am J Kidney Dis 71(3):362–370
Cao XS, Chen J, Zou JZ et al (2015) Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol 10(1):111–119
Jin W, Yan W, Li DY et al (2019) Study on the relationship between indoxyl sulfate and NLRP3 inflammasome and mir-34A expression in rats with chronic renal insufficiency. Mod Dig Interv Diagn Treat 4:343–346
Xie J, Wu YL, Huang CL (2016) Deficiency of soluble α-klotho as an independent cause of uremic cardiomyopathy. Vitam Horm 101:311–330
Yang K, Wang C, Nie L et al (2015) Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol 26(10):2434–2446
Chen J, Zhang XY, Zhang H et al (2016) Indoxyl sulfate enhance the hypermethylation of klotho and promote the process of vascular calcification in chronic kidney disease. Int J Biol Sci 12(10):1236–1246
Hung SC, Kuo KL, Huang HL et al (2016) Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int 89(3):574–585
Kamiński Tomasz W, Krystyna P, Małgorzata K et al (2017) Indoxyl sulfate–the uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol 18(1):35
He T, Xiong J, Huang Y et al (2019) Klotho restrain RIG-1/NF-κB signaling activation and monocyte inflammatory factor release under uremic condition. Life Sci 231:116570
Watanabe I, Tatebe J, Fujii T et al (2019) Prognostic significance of serum indoxyl sulfate and albumin for patients with cardiovascular disease. Int Heart J 60(1):129–135
Opdebeeck B, Maudsley S, Azmi A et al (2019) Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J Am Soc Nephrol 30(5):751–766
He X, Jiang HL, Gao FF et al (2019) Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-κB signaling pathway. Microsc Res Tech 82(12):2000–2006
Kim HY, Yoo TH, Cho JY et al (2019) Indoxyl sulfate-induced TNF-α is regulated by crosstalk between the aryl hydrocarbon receptor, NF-κB, and SOCS2 in human macrophages. FASEB J 33(10):10844–10858
Ryu JH, Jeon EY, Kim SJ (2019) Indoxyl sulfate-induced extracellular vesicles released from endothelial cells stimulate vascular smooth muscle cell proliferation by inducing transforming growth factor-beta production. J Vasc Res 56(3):129–138
Suree L (2018) Cardiotoxicity of uremic toxins: a driver of cardiorenal syndrome. Toxins (Basel) 10(9):352
Asanuma H, Chung H, Ito S et al (2019) AST-120, an adsorbent of uremic toxins, improves the pathophysiology of heart failure in conscious dogs. Cardiovasc Drugs Ther 33(3):277–286
Liu WC, Wu CC, Lim PS et al (2018) Effect of uremic toxin-indoxyl sulfate on the skeletal system. Clin Chim Acta 484:197–206
Adelibieke Y, Shimizu H, Saito S et al (2013) Indoxyl sulfate counteracts endothelial effects of erythropoietin through suppression of akt phosphorylation. Circ J 77(5):1326–1336
Ahmed Mohamed SE, Abed M, Voelkl J et al (2013) Triggering of suicidal erythrocyte death by uremic toxin indoxyl sulfate. BMC Nephrol 14:244
Asai H, Hirata J, Hirano A et al (2016) Activation of aryl hydrocarbon receptor mediates suppression of hypoxia-inducible factor-dependent erythropoietin expression by indoxyl sulfate. Am J Physiol Cell Physiol 310(2):C142-150
Wu CJ, Chen CY, Lai TS et al (2017) The role of indoxyl sulfate in renal anemia in patients with chronic kidney disease. Oncotarget 8(47):83030–83037
Dias GF, Bonan NB, Steiner TM et al (2018) Indoxyl sulfate, a uremic toxin, stimulates reactive oxygen species production and erythrocyte cell death supposedly by an organic anion transporter 2 (OAT2) and NADPH oxidase activity-dependent pathways. Toxins (Basel) 10(07):280
Hamano H, Ikeda Y, Watanabe H et al (2018) The uremic toxin indoxyl sulfate interferes with iron metabolism by regulating hepcidin in chronic kidney disease. Nephrol Dial Transplant 33(4):586–597
Wu IW, Hsu KH, Sun CY et al (2014) Oral adsorbent AST-120 potentiates the effect of erythropoietin-stimulating agents on stage 5 chronic kidney disease patients: a randomized crossover study. Nephrol Dial Transplant 29(9):1719–1727
Bataille S, Pelletier M, Sallée M et al (2017) Indole 3-acetic acid, indoxyl sulfate and paracresyl-sulfate do not influence anemia parameters in hemodialysis patients. BMC Nephrol 18(1):251
Deltombe O, Glorieux G, Marzouki S et al (2019) Selective transport of protein-bound uremic toxins in erythrocytes. Toxins (Basel) 11(7):385
Massy Z, Drueke T (2017) Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J Nephrol 30(5):629–634
Yamamoto S, Fukagawa M (2017) Uremic Toxicity and Bone in CKD. J Nephrol 30(5):623–627
BarretoFellype C, Barreto Daniela V, Canziani Maria EF et al (2014) Association between indoxyl sulfate and bone histomorphometry in pre-dialysis chronic kidney disease patients. J Bras Nefrol 36(3):289–296
Kim YH, Kwak KA, Gil HW et al (2013) Indoxyl sulfate promotes apoptosis in cultured osteoblast cells. BMC Pharmacol Toxicol 14:60
Mozar A, Louvet L, Godin C et al (2012) Indoxyl sulphate inhibits osteoclast differentiation and function. Nephrol Dial Transplant 27:2176–2181
Liao YL, Chou CC, Lee YJ (2019) The association of indoxyl sulfate with fibroblast growth factor-23 in cats with chronic kidney disease. J Vet Intern Med 33(2):686–693
Hamada-Ode K, Taniguchi Y, Shimamura Y et al (2019) Serum dickkopf-related protein 1 and sclerostin may predict the progression of chronic kidney disease in Japanese patients. Nephrol Dial Transplant 34(8):1426–1427
Desjardins L, Liabeuf S, Oliveira RB et al (2014) Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol Ther 10(4):463–470
Changchien CY, Lin YH, Cheng YC et al (2019) Indoxyl sulfate induces myotube atrophy by ROS-ERK and JNK-MAFbx cascades. Chem Biol Interact 304:43–51
Thome T, Salyers ZR, Kumar RA et al (2019) Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am J Physiol, Cell Physiol 317(4):C701–C713
Sato E, Mori T, Mishima E et al (2016) Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep 6:36618
Lin YT, Wu PH, Tsai YC et al (2019) Indoxyl sulfate induces apoptosis through oxidative stress and mitogen-activated protein kinase signaling pathway inhibition in human astrocytes. J Clin Med 8(2):191
Watanabe K, Watanabe T, Nakayama M (2014) Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology 44:184–193
Adesso S, Magnus T, Cuzzocrea S et al (2017) Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front Pharmacol 8:370
Leong Sheldon C, Sirich TL (2016) Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins (Basel) 8(12):358
Yeh YC, Huang MF, Liang SS et al (2016) Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 53:148–152
Hosoya K, Tachikawa M (2011) Roles of organic anion/cation transporters at the blood-brain and blood-cerebrospinal fluid barriers involving uremic toxins. Clin Exp Nephrol 15(4):478–485
Stinghen AE, Chillon JM, Massy ZA et al (2014) Differential effects of indoxyl sulfate and inorganic phosphate in a murine cerebral endothelial cell line (bEnd.3). Toxins (Basel) 6(6):1742–1760
Shu C, Chen XJ, Xia TY et al (2016) LC-MS/MS method for simultaneous determination of serum p-cresyl sulfate and indoxyl sulfate in patients undergoing peritoneal dialysis. Biomed Chromatogr 30(11):1782–1788
Niwa T (2010) Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr 20(5 Suppl):S2–S6
Zhang A, Rijal K, Ng Seng K et al (2017) A mass spectrometric method for quantification of tryptophan-derived uremic solutes in human serum. J Biol Methods 4(3):e75
Prokopienko Alexander J, West Raymond E, Stubbs Jason R et al (2019) Development and validation of a UHPLC-MS/MS method for measurement of a gut-derived uremic toxin panel in human serum: an application in patients with kidney disease. J Pharm Biomed Anal 174:618–624
Xiao YH, Ge LY, Zhu Q et al (2019) Determination of indoxyl sulfate and p-cresol sulfate in human plasma by ultra performance liquid chromatography-tandem mass spectrometry. J Anal Sci 2:243–246
Fushimi Y, Tatebe J, Okuda Y et al (2019) Performance evaluation of an indoxyl sulfate assay kit “NIPRO.” Clin Chem Lab Med 57(11):1770–1776
Matsuo K, Yamamoto S, Wakamatsu T et al (2015) Increased proinflammatory cytokine production and decreased cholesterol efflux due to downregulation of ABCG1 in macrophages exposed to indoxyl sulfate. Toxins (Basel) 7(8):3155–3166
Itoh Y, Ezawa A, Kikuchi K et al (2012) Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem 403(7):1841–1850
Yamamoto SGR, Zuo YQ, Ma J et al (2011) Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol Dial Transplant 26(8):2491–2497
Yang K, Xu X, Nie L et al (2015) Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol Lett 234(2):110–119
Hideki F, Fuyuhiko N, Sumie G et al (2009) Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant 24(7):2089–2095
Lekawanvijit S, Kompa AR, Manabe M et al (2012) Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS ONE 7(7):e41281
Savira F, Cao L, Wang I et al (2017) Apoptosis signal-regulating kinase 1 inhibition attenuates cardiac hypertrophy and cardiorenal fibrosis induced by uremic toxins: implications for cardiorenal syndrome. PLoS ONE 12(11):e0187459
Iwasaki Y, Kazama JJ, Yamato H et al (2013) Accumulated uremic toxins attenuate bone mechanical properties in rats with chronic kidney disease. Bone 57(2):477–483
Sirich Tammy L, Fong K, Larive B et al (2017) Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the frequent hemodialysis network daily trial. Kidney Int 91(5):1186–1192
Pavlenko D, van Geffen E, van Steenbergen MJ et al (2016) New low-flux mixed matrix membranes that offer superior removal of protein-bound toxins from human plasma. Sci Rep 6:34429
Madero M, Cano KB, Campos I et al (2019) Removal of protein-bound uremic toxins during hemodialysis using a binding competitor. Clin J Am Soc Nephrol 14(3):394–402
Maheshwari V, Thijssen S, Tao X et al (2019) In silico comparison of protein-bound uremic toxin removal by hemodialysis, hemodiafiltration, membrane adsorption, and binding competition. Sci Rep 9(1):909
Suguru Y, Mami S, Yoko S et al (2018) Adsorption of protein-bound uremic toxins through direct hemoperfusion with hexadecyl-immobilized cellulose beads in patients undergoing hemodialysis. Artif Organs 42(1):88–93
Marieke S, Sven T-B, Tobias B et al (2019) A bifunctional adsorber particle for the removal of hydrophobic uremic toxins from whole blood of renal failure patients. Toxins (Basel) 11(7):389
Cai YJ, Sun XG, Sang XP et al (2019) Effect of Tong Fu Hua Zhuo Liang Xue prescription on elimination of indoxyl sulfate in rats with chronic renal failure. Acta Chinese Med Pharmacol 47(01):30–33
Bennis Y, Cluet Y, Titeca-Beauport D et al (2019) The effect of sevelamer on serum levels of gut-derived uremic toxins: results from in vitro experiments and a multicenter, double-blind, placebo-controlled randomized clinical trial. Toxins (Basel) 11(5):279
Asai M, Kumakura S, Kikuchi M (2019) Review of the efficacy of AST-120 (KREMEZIN) on renal function in chronic kidney disease patients. Ren Fail 41(1):47–56
Pavlenko D, Giasafaki D, Charalambopoulou G et al (2017) Carbon adsorbents with dual porosity for efficient removal of uremic toxins and cytokines from human plasma. Sci Rep 7(1):14914
Anraku M, Tabuchi R, Ifuku S et al (2017) An oral absorbent, surface-deacetylated chitin nano-fiber ameliorates renal injury and oxidative stress in 5/6 nephrectomized rats. Carbohydr Polym 161:21–25
Sandeman SR, Zheng Y, Ingavle GC et al (2017) A haemocompatible and scalable nanoporous adsorbent monolith synthesised using a novel lignin binder route to augment the adsorption of poorly removed uraemic toxins in haemodialysis. Biomed Mater 12(3):035001
Okishima A, Koide H, Hoshino Y et al (2019) Design of synthetic polymer nanoparticles specifically capturing indole, a small toxic molecule. Biomacromol 20(4):1644–1654
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Funding
Foundation item: The project of introducing overseas students of Hebei province, C20210357, Yan Gao.
Author information
Authors and Affiliations
Contributions
YG and YL conceived of the study, and XD and QW participated in its design and Qian Wang helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
All of the authors had no any personal, financial, commercial or academic conflicts of interest separately.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Y., Li, Y., Duan, X. et al. Research progress on the relationship between IS and kidney disease and its complications. Int Urol Nephrol 54, 2881–2890 (2022). https://doi.org/10.1007/s11255-022-03209-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03209-1